Pharmacokinetics of buprenorphine in pregnant sheep after intravenous injection
- PMID: 33619904
- PMCID: PMC7899927
- DOI: 10.1002/prp2.726
Pharmacokinetics of buprenorphine in pregnant sheep after intravenous injection
Abstract
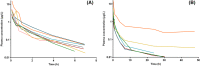
Buprenorphine is a semi-synthetic opioid, widely used in the maintenance treatment for opioid-dependent pregnant women. Limited data exist on the pharmacokinetics of buprenorphine in pregnancy. We conducted a pharmacokinetic study to determine the pharmacokinetics of intravenous buprenorphine in pregnant sheep. Fourteen pregnant sheep in late gestation received 10 µg/kg of buprenorphine as an intravenous bolus injection. Plasma samples were collected up to 48 h after administration. Buprenorphine and its metabolite, norbuprenorphine, were quantified from plasma using a LC/MS/MS method, with lower limits of quantification of 0.01 µg/L and 0.04 µg/L for buprenorphine and norbuprenorphine, respectively. The pharmacokinetic parameters were calculated using noncompartmental analysis. The pharmacokinetic parameters, median (minimum-maximum), were Cmax 4.31 µg/L (1.93-15.5), AUCinf 2.89 h*µg/L (1.72-40.2), CL 3.39 L/h/kg (0.25-6.02), terminal t½ 1.75 h (1.07-31.0), Vss 8.04 L/kg (1.05-49.3). Norbuprenorphine was undetected in all plasma samples. The median clearance in pregnant sheep was higher than previously reported for nonpregnant sheep and human (male) subjects. Our sensitive analytical method was able to detect long terminal half-lives for six subjects, and a wide between-subject variability in the study population. Significance statement: Buprenorphine is widely used for the treatment of opioid use disorder in pregnancy. However, limited data exist on the pharmacokinetics of buprenorphine during pregnancy. As this type of study cannot be done in humans due to ethical reasons, we conducted a study in pregnant sheep. This study provides pharmacokinetic data on buprenorphine in pregnant sheep and helps us to understand the pharmacokinetics of the drug in humans.
Keywords: buprenorphine; pharmacokinetics; pregnancy; sheep.
© 2021 The Authors. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Gestational changes in buprenorphine exposure: A physiologically-based pharmacokinetic analysis.Br J Clin Pharmacol. 2018 Sep;84(9):2075-2087. doi: 10.1111/bcp.13642. Epub 2018 Jun 21. Br J Clin Pharmacol. 2018. PMID: 29873094 Free PMC article.
-
Dose-adjusted plasma concentrations of sublingual buprenorphine are lower during than after pregnancy.Am J Obstet Gynecol. 2017 Jan;216(1):64.e1-64.e7. doi: 10.1016/j.ajog.2016.09.095. Epub 2016 Sep 26. Am J Obstet Gynecol. 2017. PMID: 27687214 Free PMC article.
-
MATERNAL AND FETAL BUPRENORPHINE PHARMACOKINETICS IN PREGNANT SHEEP DURING TRANSDERMAL PATCH DOSING: Buprenorphine pharmacokinetics in pregnant sheep.Eur J Pharm Sci. 2021 Oct 1;165:105936. doi: 10.1016/j.ejps.2021.105936. Epub 2021 Jul 14. Eur J Pharm Sci. 2021. PMID: 34273481
-
Pharmacokinetics of the combination tablet of buprenorphine and naloxone.Drug Alcohol Depend. 2003 May 21;70(2 Suppl):S39-47. doi: 10.1016/s0376-8716(03)00058-9. Drug Alcohol Depend. 2003. PMID: 12738349 Review.
-
Buprenorphine-mediated transition from opioid agonist to antagonist treatment: state of the art and new perspectives.Curr Drug Abuse Rev. 2012 Mar;5(1):52-63. doi: 10.2174/1874473711205010052. Curr Drug Abuse Rev. 2012. PMID: 22280332 Free PMC article. Review.
Cited by
-
Animal models of postpartum hemorrhage.Lab Anim (NY). 2024 Apr;53(4):93-106. doi: 10.1038/s41684-024-01349-8. Epub 2024 Mar 25. Lab Anim (NY). 2024. PMID: 38528231 Review.
-
Buprenorphine Pharmacodynamics: A Bridge to Understanding Buprenorphine Clinical Benefits.Drugs. 2025 Feb;85(2):215-230. doi: 10.1007/s40265-024-02128-y. Epub 2025 Jan 28. Drugs. 2025. PMID: 39873915 Review.
-
An Exploration of Analgesia Options for Australian Sheep.Animals (Basel). 2024 Mar 22;14(7):990. doi: 10.3390/ani14070990. Animals (Basel). 2024. PMID: 38612229 Free PMC article. Review.
References
-
- Huang P, Kehner GB, Cowan A, Liu‐Chen LY. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther. 2001;297(2):688‐695. - PubMed
-
- Clarke’s Analysis of Drugs and Poisons: Buprenorphine. https://www.medicinescomplete.com/#/content/clarke/c‐d1e168521?hspl=bupr.... Published 2019. Accessed March 10, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

