Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19
- PMID: 33620086
- PMCID: PMC8407425
- DOI: 10.1002/14651858.CD013665.pub2
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19
Update in
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
Abstract
Background: The clinical implications of SARS-CoV-2 infection are highly variable. Some people with SARS-CoV-2 infection remain asymptomatic, whilst the infection can cause mild to moderate COVID-19 and COVID-19 pneumonia in others. This can lead to some people requiring intensive care support and, in some cases, to death, especially in older adults. Symptoms such as fever, cough, or loss of smell or taste, and signs such as oxygen saturation are the first and most readily available diagnostic information. Such information could be used to either rule out COVID-19, or select patients for further testing. This is an update of this review, the first version of which published in July 2020.
Objectives: To assess the diagnostic accuracy of signs and symptoms to determine if a person presenting in primary care or to hospital outpatient settings, such as the emergency department or dedicated COVID-19 clinics, has COVID-19.
Search methods: For this review iteration we undertook electronic searches up to 15 July 2020 in the Cochrane COVID-19 Study Register and the University of Bern living search database. In addition, we checked repositories of COVID-19 publications. We did not apply any language restrictions.
Selection criteria: Studies were eligible if they included patients with clinically suspected COVID-19, or if they recruited known cases with COVID-19 and controls without COVID-19. Studies were eligible when they recruited patients presenting to primary care or hospital outpatient settings. Studies in hospitalised patients were only included if symptoms and signs were recorded on admission or at presentation. Studies including patients who contracted SARS-CoV-2 infection while admitted to hospital were not eligible. The minimum eligible sample size of studies was 10 participants. All signs and symptoms were eligible for this review, including individual signs and symptoms or combinations. We accepted a range of reference standards.
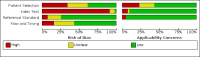
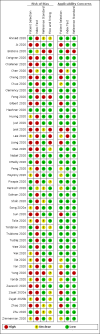
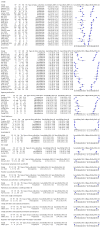
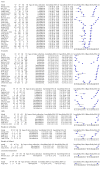
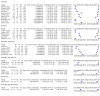
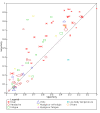
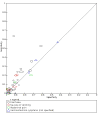
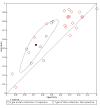
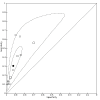
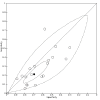
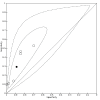
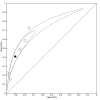
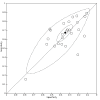
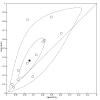
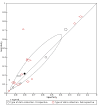
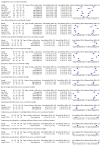
Data collection and analysis: Pairs of review authors independently selected all studies, at both title and abstract stage and full-text stage. They resolved any disagreements by discussion with a third review author. Two review authors independently extracted data and resolved disagreements by discussion with a third review author. Two review authors independently assessed risk of bias using the Quality Assessment tool for Diagnostic Accuracy Studies (QUADAS-2) checklist. We presented sensitivity and specificity in paired forest plots, in receiver operating characteristic space and in dumbbell plots. We estimated summary parameters using a bivariate random-effects meta-analysis whenever five or more primary studies were available, and whenever heterogeneity across studies was deemed acceptable.
Main results: We identified 44 studies including 26,884 participants in total. Prevalence of COVID-19 varied from 3% to 71% with a median of 21%. There were three studies from primary care settings (1824 participants), nine studies from outpatient testing centres (10,717 participants), 12 studies performed in hospital outpatient wards (5061 participants), seven studies in hospitalised patients (1048 participants), 10 studies in the emergency department (3173 participants), and three studies in which the setting was not specified (5061 participants). The studies did not clearly distinguish mild from severe COVID-19, so we present the results for all disease severities together. Fifteen studies had a high risk of bias for selection of participants because inclusion in the studies depended on the applicable testing and referral protocols, which included many of the signs and symptoms under study in this review. This may have especially influenced the sensitivity of those features used in referral protocols, such as fever and cough. Five studies only included participants with pneumonia on imaging, suggesting that this is a highly selected population. In an additional 12 studies, we were unable to assess the risk for selection bias. This makes it very difficult to judge the validity of the diagnostic accuracy of the signs and symptoms from these included studies. The applicability of the results of this review update improved in comparison with the original review. A greater proportion of studies included participants who presented to outpatient settings, which is where the majority of clinical assessments for COVID-19 take place. However, still none of the studies presented any data on children separately, and only one focused specifically on older adults. We found data on 84 signs and symptoms. Results were highly variable across studies. Most had very low sensitivity and high specificity. Only cough (25 studies) and fever (7 studies) had a pooled sensitivity of at least 50% but specificities were moderate to low. Cough had a sensitivity of 67.4% (95% confidence interval (CI) 59.8% to 74.1%) and specificity of 35.0% (95% CI 28.7% to 41.9%). Fever had a sensitivity of 53.8% (95% CI 35.0% to 71.7%) and a specificity of 67.4% (95% CI 53.3% to 78.9%). The pooled positive likelihood ratio of cough was only 1.04 (95% CI 0.97 to 1.11) and that of fever 1.65 (95% CI 1.41 to 1.93). Anosmia alone (11 studies), ageusia alone (6 studies), and anosmia or ageusia (6 studies) had sensitivities below 50% but specificities over 90%. Anosmia had a pooled sensitivity of 28.0% (95% CI 17.7% to 41.3%) and a specificity of 93.4% (95% CI 88.3% to 96.4%). Ageusia had a pooled sensitivity of 24.8% (95% CI 12.4% to 43.5%) and a specificity of 91.4% (95% CI 81.3% to 96.3%). Anosmia or ageusia had a pooled sensitivity of 41.0% (95% CI 27.0% to 56.6%) and a specificity of 90.5% (95% CI 81.2% to 95.4%). The pooled positive likelihood ratios of anosmia alone and anosmia or ageusia were 4.25 (95% CI 3.17 to 5.71) and 4.31 (95% CI 3.00 to 6.18) respectively, which is just below our arbitrary definition of a 'red flag', that is, a positive likelihood ratio of at least 5. The pooled positive likelihood ratio of ageusia alone was only 2.88 (95% CI 2.02 to 4.09). Only two studies assessed combinations of different signs and symptoms, mostly combining fever and cough with other symptoms. These combinations had a specificity above 80%, but at the cost of very low sensitivity (< 30%).
Authors' conclusions: The majority of individual signs and symptoms included in this review appear to have very poor diagnostic accuracy, although this should be interpreted in the context of selection bias and heterogeneity between studies. Based on currently available data, neither absence nor presence of signs or symptoms are accurate enough to rule in or rule out COVID-19. The presence of anosmia or ageusia may be useful as a red flag for COVID-19. The presence of fever or cough, given their high sensitivities, may also be useful to identify people for further testing. Prospective studies in an unselected population presenting to primary care or hospital outpatient settings, examining combinations of signs and symptoms to evaluate the syndromic presentation of COVID-19, are still urgently needed. Results from such studies could inform subsequent management decisions.
Copyright © 2021 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
Thomas Struyf: none known
Jonathan J Deeks: none known
Jacqueline Dinnes: none known
Yemisi Takwoingi: none known
Clare Davenport: none known
Mariska MG Leeflang: none known
René Spijker: the Dutch Cochrane Centre (DCC) has received grants for performing commissioned systematic reviews. In no situation did the commissioner have any influence on the results of the work.
Lotty Hooft: none known
Devy Emperador: is employed by FIND. FIND is a global non‐for profit product development partnership and WHO Diagnostic Collaboration Centre. It is FIND’s role to accelerate access to high quality diagnostic tools for low resource settings and this is achieved by supporting both R&D and access activities for a wide range of diseases, including COVID‐19. FIND has several clinical research projects to evaluate multiple new diagnostic tests against published Target Product Profiles that have been defined through consensus processes. These studies are for diagnostic products developed by private sector companies who provide access to know‐how, equipment/reagents, and contribute through unrestricted donations as per FIND policy and external SAC review.
Julie Domen: none known
Sebastiaan Horn: none known
Ann Van den Bruel: none known
Figures





















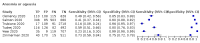
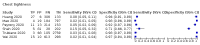


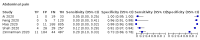
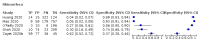
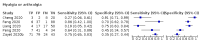
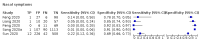
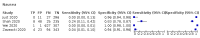
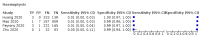
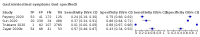
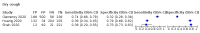
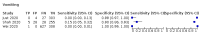
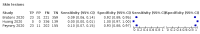
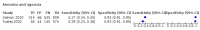
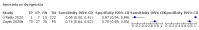
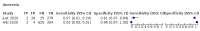
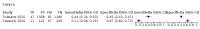
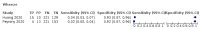
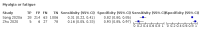
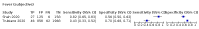
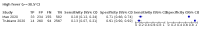
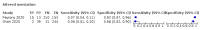
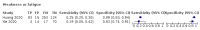
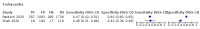
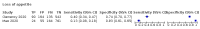
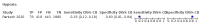
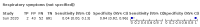
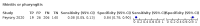
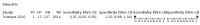
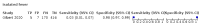
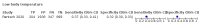
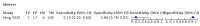
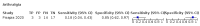
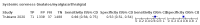
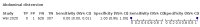
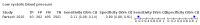
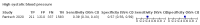
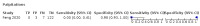
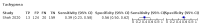
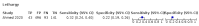
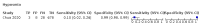
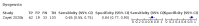
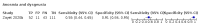
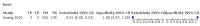
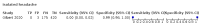
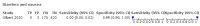
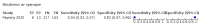
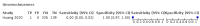
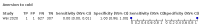
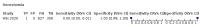
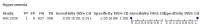
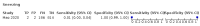
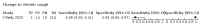
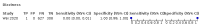
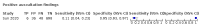
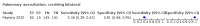
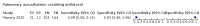
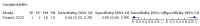
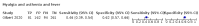
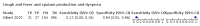
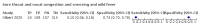
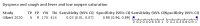
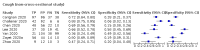
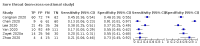
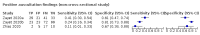
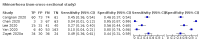
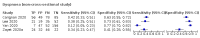
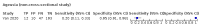
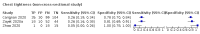
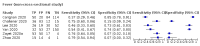
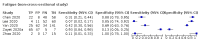
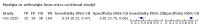
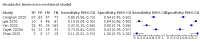
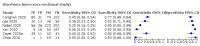
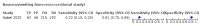
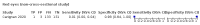
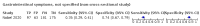
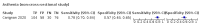
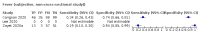
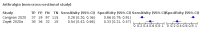
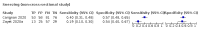
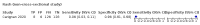
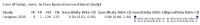
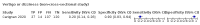
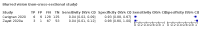
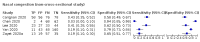
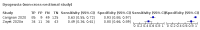
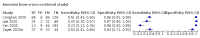
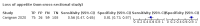
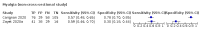
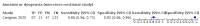
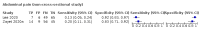
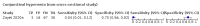
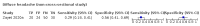
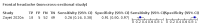
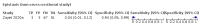
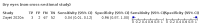
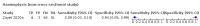
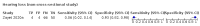
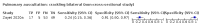
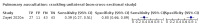
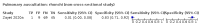
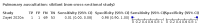
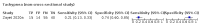
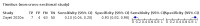
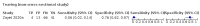
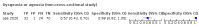
Update of
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease.Cochrane Database Syst Rev. 2020 Jul 7;7(7):CD013665. doi: 10.1002/14651858.CD013665. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Feb 23;2:CD013665. doi: 10.1002/14651858.CD013665.pub2. PMID: 32633856 Free PMC article. Updated.
References
References to studies included in this review
Ahmed 2020 {published data only}
-
- Ahmed SM, Shah RU, Bale M, Peacock JB, Berger B, Brown A, et al. Comprehensive testing highlights racial, ethnic, and age disparities in the COVID-19 outbreak. medRxiv [Preprint] 2020. [DOI: ]
Ai 2020 {published data only}
-
- Ai JW, Zhang HC, Xu T, Wu J, Zhu M, Yu YQ, et al. Optimizing diagnostic strategy for novel coronavirus pneumonia, a multi-center study in Eastern China. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.02.13.20022673] - DOI
Brotons 2020 {published data only}
-
- Brotons C, Serrano J, Fernandez D, Garcia-Ramos C, Ichazo B, Lemaire J, et al. Seroprevalence against COVID-19 and follow-up of suspected cases in primary health care in Spain. medRxiv [Preprint] 2020. [DOI: ]
Carignan 2020 {published data only}
Challener 2020 {published data only}
Chen 2020 {published data only}
Cheng 2020 {published data only}
Chua 2020 {published data only}
Clemency 2020 {published data only}
Feng 2020 {published data only}
Gilbert 2020 {published data only}
-
- Gilbert A, Brasseur E, Petit M, Donneau AF, Diep AN, Hetzel Campbell S, et al. Immersion in an emergency department triage center during the COVID-19 outbreak: first report of the Liège University hospital experience. Acta Clinica Belgica 2020;Jun(12):1-7. - PubMed
Haehner 2020 {published data only}
Huang 2020 {published data only}
Just 2020 {published data only}
Leal 2020 {published data only}
Lee 2020 {published data only}
Liang 2020 {published data only}
-
- Liang Y, Liang J, Zhou Q, Li X, Lin F, Deng Z, et al. Prevalence and clinical features of 2019 novel coronavirus disease (COVID-19) in the Fever Clinic of a teaching hospital in Beijing: a single-center, retrospective study. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.02.25.20027763] - DOI
Mao 2020 {published data only}
Nobel 2020 {published data only}
O'Reilly 2020 {published data only}
-
- O'Reilly GM, Mitchell RD, Rajiv P, Wu J, Brennecke H, Brichko L, et al. Epidemiology and clinical features of emergency department patients with suspected COVID-19: initial results from the COVID-19 Emergency Department Quality Improvement Project (COVED-1). Emergency Medicine Australasia 2020;32(4):638-45. - PubMed
Peng 2020 {published data only}
-
- Peng L, Liu KY, Xue F, Miao YF, Tu PA, Zhou C. Improved early recognition of coronavirus disease-2019 (COVID-19): single-center data from a Shanghai screening hospital. Archives of Iranian Medicine 2020;23(4):272-6. - PubMed
Peyrony 2020 {published data only}
Pisapia 2020 {published data only}
-
- Pisapia R, Pisaturo M, Fusco FM, Parrella G, Iodice V, Tambaro O, et al. Differences among confirmed and not-confirmed COVID-19 patients at "D.Cotugno" hospital, Naples (Italy): what we learned from first suspected cases? Infezioni in Medicina 2020;28 Suppl 1:84-8. - PubMed
Rentsch 2020 {published data only}
-
- Rentsch CT, Kidwai-Khan F, Tate JP, Park LS, King JT, Skanderson M, et al. Covid-19 testing, hospital admission, and intensive care among 2,026,227 United States veterans aged 54-75 years. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.04.09.20059964] - DOI
Salmon 2020 {published data only}
Shah 2020 {published data only}
Song 2020a {published data only}
-
- Song CY, Xu J, He JQ, Lu YQ. COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.03.05.20031906] - DOI
Sun 2020 {published data only}
Tolia 2020 {published data only}
Tordjman 2020 {published data only}
Trubiano 2020 {published data only}
Tudrej 2020 {published data only}
Wee 2020 {published data only}
Wei 2020 {published data only}
Xie 2020 {published data only}
Yan 2020 {published data only}
Yang 2020 {unpublished data only}
Yombi 2020 {published data only}
Zavascki 2020 {published data only}
-
- Zavascki AP, Gazzana MB, Bidart JP, Fernandes PS, Galiotto A, Kawski CT, et al. Development of a predictive score for COVID-19 diagnosis based on demographics and symptoms in patients attended at a dedicated screening unit. medRxiv [Preprint] 2020. [DOI: ]
Zayet 2020a {published data only}
Zayet 2020b {published data only}
Zhao 2020 {published data only}
Zhu 2020 {published data only}
Zimmerman 2020 {published data only}
References to studies excluded from this review
Guan 2020 {published data only}
-
- Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.02.06.20020974] - DOI
Soares 2020 {published data only}
-
- Soares F, Villavicencio A, Anzanello MJ, Fogliatto FS, Idiart M, Stevenson M. A novel high specificity COVID-19 screening method based on simple blood exams and artificial intelligence. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.04.10.20061036] - DOI
Song 2020b {published data only}
References to ongoing studies
ChiCTR2000029462 {published data only}
-
- ChiCTR2000029462. Study for clinical characteristics and distribution of TCM syndrome of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=48922 (first received 27 April 2020).
ChiCTR2000029734 {published data only}
-
- ChiCTR2000029734. Epidemiological investigation and clinical characteristics analysis of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=48868 (first received 27 April 2020).
ChiCTR2000029770 {published data only}
-
- ChiCTR2000029770. Study for epidemiology, diagnosis and treatment of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/hvshowproject.aspx?id=23744 (first received 27 April 2020).
ChiCTR2000029839 {published data only}
-
- ChiCTR2000029839. An observational study on the clinical characteristics, treatment and outcome of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=49439 (first received 27 April 2020).
ChiCTR2000029865 {published data only}
-
- ChiCTR2000029865. Descriptive study on the clinical characteristics and outcomes of novel coronavirus pneumonia (COVID-19) in cardiovascular patients. www.chictr.org.cn/showproj.aspx?proj=49545 (first received 27 April 2020).
ChiCTR2000029866 {published data only}
-
- ChiCTR2000029866. Early warning prediction of patients with severe novel coronavirus pneumonia (COVID-19) based on multiomics. www.chictr.org.cn/showproj.aspx?proj=49519 (first received 27 April 2020).
ChiCTR2000029959 {published data only}
-
- ChiCTR2000029959. Clinical observation and research of severe acute respiratory syndrome coronavirus 2(COVID-19) infection in perinatal newborns. www.chictr.org.cn/showproj.aspx?proj=49636 (first received 27 April 2020).
ChiCTR 2000030096 {published data only}
-
- ChiCTR 2000030096. Study for establishment of correlation between virological dynamics and clinical features in novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showprojen.aspx?proj=49794 (first received 27 April 2020).
ChiCTR2000030256 {published data only}
-
- ChiCTR2000030256. Epidemiological and clinical characteristics of COVID-19: a large-scale investigation in epicenter Wuhan, China. www.chictr.org.cn/showproj.aspx?proj=50078 (first received 27 April 2020).
ChiCTR2000030327 {published data only}
-
- ChiCTR2000030327. Analysis of clinical characteristics of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50214 (first received 27 April 2020).
ChiCTR2000030363 {published data only}
-
- ChiCTR2000030363. Novel coronavirus infected disease (COVID-19) in children: epidemiology, clinical features and treatment outcome. www.chictr.org.cn/showproj.aspx?proj=49984 (first received 27 April 2020).
ChiCTR2000030387 {published data only}
-
- ChiCTR2000030387. Clinical observation and research of multiple organs injury in severe patients with novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50329 (first received 27 April 2020).
ChiCTR2000030464 {published data only}
-
- ChiCTR2000030464. Study for the clinical characteristics of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50382 (first received 27 April 2020).
ChiCTR2000030491 {published data only}
-
- ChiCTR2000030491. A medical records based study for comparing differences of clinical features and outcomes of novel coronavirus pneumonia (COVID-19) patients between Sichuan Province and Wuhan City. www.chictr.org.cn/hvshowproject.aspx?id=23102 (first received 27 April 2020).
ChiCTR2000030519 {published data only}
-
- ChiCTR2000030519. Study for the clinical characteristics and digestive system damage of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50604 (first received 27 April 2020).
ChiCTR2000030544 {published data only}
-
- ChiCTR2000030544. Study for the risk factors of critically ill patients with novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50134 (first received 27 April 2020).
ChiCTR 2000030679 {published data only}
-
- ChiCTR 2000030679. Cohort study of novel coronavirus infected diseases (COVID-19) in children. www.chictr.org.cn/hvshowproject.aspx?id=23417 (first received 27 April 2020).
ChiCTR2000030707 {published data only}
-
- ChiCTR2000030707. Retrospective study on novel coronavirus pneumonia (COVID-19) in Tibetan Plateau. www.chictr.org.cn/showproj.aspx?proj=50160 (first received 27 April 2020).
ChiCTR2000030722 {published data only}
-
- ChiCTR2000030722. Auscultatory characteristics of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50338 (first received 27 April 2020).
ChiCTR2000030739 {published data only}
-
- ChiCTR2000030739. Exploration of the clinical characteristics of patients with novel coronavirus pneumonia (COVID-19) and its differences from patients with severe influenza A and MERS. www.chictr.org.cn/showproj.aspx?proj=50896 (first received 27 April 2020).
ChiCTR2000030755 {published data only}
-
- ChiCTR2000030755. A medical records based study for characteristics, prognosis of elderly patients with novel coronavirus pneumonia (COVID-19) in Wuhan area. www.chictr.org.cn/hvshowproject.aspx?id=23554 (first received 27 April 2020).
ChiCTR2000030778 {published data only}
-
- ChiCTR2000030778. A medical records based study for epidemic and clinical features of novel coronavirus pneumonia (COVID-19) in Ningbo First Hospital. www.chictr.org.cn/hvshowproject.aspx?id=23642 (first received 27 April 2020).
ChiCTR2000030784 {published data only}
-
- ChiCTR2000030784. A study for clinical characteristics of novel coronavirus pneumonia (COVID-19) patients follow-up in Guangxi. www.chictr.org.cn/showproj.aspx?proj=50307 (first received 27 April 2020).
ChiCTR2000030796 {published data only}
-
- ChiCTR2000030796. Clinical characteristics and treatment of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50991 (first received 27 April 2020).
ChiCTR 2000030798 {published data only}
-
- ChiCTR 2000030798. A medical records based study for clinical characteristics of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/hvshowproject.aspx?id=23687 (first received 27 April 2020).
ChiCTR2000030803 {published data only}
-
- ChiCTR2000030803. Collection and analysis of clinical data in severe and critically ill patients with novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=51007 (first received 27 April 2020).
ChiCTR2000030807 {published data only}
-
- ChiCTR2000030807. Clinical characteristics and prognosis of cancer patients with novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=51019 (first received 27 April 2020).
ChiCTR2000030818 {unpublished data only}
-
- ChiCTR2000030818. A medical records based study for the value of Lymphocyte subsets in the diagnose and treatment. www.chictr.org.cn/hvshowproject.aspx?id=23742 (first received 27 April 2020).
ChiCTR2000030819 {published data only (unpublished sought but not used)}
-
- ChiCTR2000030819. Retrospective analysis of digestive system symptoms in 600 cases of novel coronavirus pneumonia (COVID-19) in Guanggu district, Wuhan. www.chictr.org.cn/showproj.aspx?proj=51039 2020.
ChiCTR2000030834 {published data only}
-
- ChiCTR2000030834. Epidemiological characteristics and antibody levels of novel coronavirus pneumonia (COVID-19) of pediatric medical staff working in quarantine area. www.chictr.org.cn/showproj.aspx?proj=51047 (frist received 27 April 2020).
ChiCTR2000030854 {published data only}
-
- ChiCTR2000030854. A clinical multicenter study for the occurrence, development and prognosis of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showprojen.aspx?proj=51083 (first received 27 April 2020).
ChiCTR2000030858 {published data only}
-
- ChiCTR2000030858. Clinical characteristics and outcomes of 483 mild patients with novel coronavirus pneumonia (COVID-19) in Wuhan, China during the outbreak: a single-center, retrospective study from the mobile cabin hospital. www.chictr.org.cn/showproj.aspx?proj=51097 (first received 27 April 2020).
ChiCTR2000030863 {published data only}
-
- ChiCTR2000030863. Clinical and CT imaging characteristics of novel coronavirus pneumonia (COVID-19): an multicenter cohort study. www.chictr.org.cn/showproj.aspx?proj=50767 (first received 27 April 2020).
NCT04270383 {published data only}
-
- NCT04270383. Clinical characteristics and long-term prognosis of 2019-nCoV infection in children. clinicaltrials.gov/ct2/show/NCT04270383 (first received 17 February 2020).
NCT04279782 {published data only}
-
- NCT04279782. Clinical features of suspected and confirmed patients of 2019 novel coronavirus infection. www.clinicaltrials.gov/ct2/show/NCT04279782 (first received 27 April 2020).
NCT04279899 {published data only}
-
- NCT04279899. The investigation of the neonates with or with risk of COVID-19. clinicaltrials.gov/ct2/show/NCT04279899 (first received 27 April 2020).
NCT04285801 {published data only}
-
- NCT04285801. Critically ill patients with COVID-19 in Hong Kong: a multicentre observational cohort study. clinicaltrials.gov/ct2/show/NCT04285801 (first received 27 April 2020).
NCT04292327 {published data only}
-
- NCT04292327. Clinical progressive characteristics and treatment effects of 2019-novel coronavirus. clinicaltrials.gov/ct2/show/NCT04292327 (first received 27 April 2020).
NCT04292964 {published data only}
-
- NCT04292964. Prognostic factors of patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04292964 (first received 27 April 2020).
NCT04315870 {published data only}
-
- NCT04315870. Clinical characteristics of coronavirus disease 2019 (COVID-19) in pregnancy: the Italian Registry on coronavirus in pregnancy. clinicaltrials.gov/ct2/show/NCT04315870 (first received 20 March 2020).
Additional references
Bossuyt 2015
Deeks 2020a
-
- Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S, et al, Cochrane COVID-19 Diagnostic Test Accuracy Group. Antibody tests for identification of current and past infection with SARS‐CoV‐2. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD013652. [DOI: 10.1002/14651858.CD013652] - DOI - PMC - PubMed
Dinnes 2020
-
- Dinnes J, Deeks JJ, Adriano A, Berhane S, Davenport C, Dittrich S, et al, Cochrane COVID-19 Diagnostic Test Accuracy Group. Rapid, point‐of‐care antigen and molecular‐based tests for diagnosis of SARS‐CoV‐2 infection. Cochrane Database of Systematic Reviews 2020, Issue 8. Art. No: CD013705. [DOI: 10.1002/14651858.CD013705] - DOI - PMC - PubMed
Griffith 2020
Islam 2020
-
- Islam N, Salameh J-P, Leeflang MM, Hooft L, McGrath TA, Pol CB, et al, Cochrane COVID-19 Diagnostic Test Accuracy Group. Thoracic imaging tests for the diagnosis of COVID‐19. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD013639. [DOI: 10.1002/14651858.CD013639.pub3] - DOI - PubMed
Jaeschke 1994
-
- Jaeschke R, Guyatt GH, Sackett DL. Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA 1994;271(9):703-7. - PubMed
Macaskill 2013
-
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration, 2013. Available from srdta.cochrane.org.
McInnes 2020
Moher 2009
R 2020 [Computer program]
-
- R core team R Foundation for Statistical Computing. Version 3.5.1. Vienna, Austria: R core team, 2020.
Review Manager 2020 [Computer program]
-
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Rutjes 2006
Stegeman 2020
Van den Bruel 2010
-
- Van den Bruel A, Haj-Hassan T, Thompson M, Buntinx F, Mant D. Diagnostic value of clinical features at presentation to identify serious infection in children in developed countries: a systematic review. Lancet 2010;375(9717):834-45. - PubMed
Whiting 2011
-
- Whiting PF, Rutjes AW, Westwood ME, Mallett S Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. - PubMed
References to other published versions of this review
Deeks 2020b
-
- Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, Spijker R, et al. Diagnosis of SARS-CoV-2 infection and COVID-19: accuracy of signs and symptoms; molecular, antigen, and antibody tests; and routine laboratory markers. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD013596. [DOI: 10.1002/14651858.CD013596] - DOI
Struyf 2020
-
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang M, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID‐19 disease. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665] - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

