High versus low medium chain triglyceride content of formula for promoting short-term growth of preterm infants
- PMID: 33620090
- PMCID: PMC8094384
- DOI: 10.1002/14651858.CD002777.pub2
High versus low medium chain triglyceride content of formula for promoting short-term growth of preterm infants
Abstract
Background: In-hospital growth of preterm infants remains a challenge in clinical practice. The high nutrient demands of preterm infants often lead to growth faltering. For preterm infants who cannot be fed maternal or donor breast milk or may require supplementation, preterm formulas with fat in the form of medium chain triglycerides (MCTs) or long chain triglycerides (LCTs) may be chosen to support nutrient utilization and to improve growth. MCTs are easily accessible to the preterm infant with an immature digestive system, and LCTs are beneficial for central nervous system development and visual function. Both have been incorporated into preterm formulas in varying amounts, but their effects on the preterm infant's short-term growth remain unclear. This is an update of a review originally published in 2002, then in 2007.
Objectives: To determine the effects of formula containing high as opposed to low MCTs on early growth in preterm infants fed a diet consisting primarily of formula. SEARCH METHODS: We used the standard search strategy of Cochrane Neonatal to search Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 8), in the Cochrane Library; Ovid MEDLINE Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily, and Ovid MEDLINE(R); MEDLINE via PubMed for the previous year; and Cumulative Index to Nursing and Allied Health Literature (CINAHL), on 16 September 2020. We also searched clinical trials databases and the reference lists of retrieved articles for randomized controlled trials (RCTs) and quasi-RCTs.
Selection criteria: We included all randomized and quasi-randomized trials comparing the effects of feeding high versus low MCT formula (for a minimum of five days) on the short-term growth of preterm (< 37 weeks' gestation) infants. We defined high MCT formula as 30% or more by weight, and low MCT formula as less than 30% by weight. The infants must be on full enteral diets, and the allocated formula must be the predominant source of nutrition.
Data collection and analysis: The review authors assessed each study's quality and extracted data on growth parameters as well as adverse effects from included studies. All data used in analysis were continuous; therefore, mean differences with 95% confidence intervals were reported. We used the GRADE approach to assess the certainty of evidence.
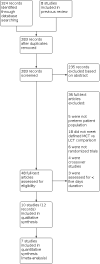
Main results: We identified 10 eligible trials (253 infants) and extracted relevant growth data from 7 of these trials (136 infants). These studies were found to provide evidence of very low to low certainty. Risk of bias was noted, as few studies described specific methods for random sequence generation, allocation concealment, or blinding. We found no evidence of differences in short-term growth parameters when high and low MCT formulas were compared. As compared to low MCT formula, preterm infants fed high MCT formula showed little to no difference in weight gain velocity (g/kg/d) during the intervention, with a typical mean difference (MD) of -0.21 g/kg/d (95% confidence interval (CI) -1.24 to 0.83; 6 studies, 118 infants; low-certainty evidence). The analysis for weight gain (g/d) did not show evidence of differences, with an MD of 0.00 g/d (95% CI -5.93 to 5.93; 1 study, 18 infants; very low-certainty evidence), finding an average weight gain of 20 ± 5.9 versus 20 ± 6.9 g/d for high and low MCT groups, respectively. We found that length gain showed no difference between low and high MCT formulas, with a typical MD of 0.10 cm/week (95% CI -0.09 to 0.29; 3 studies, 61 infants; very low-certainty evidence). Head circumference gain also showed little to no difference during the intervention period, with an MD of -0.04 cm/week (95% CI -0.17 to 0.09; 3 studies, 61 infants; low-certainty evidence). Two studies reported skinfold thickness with different measurement definitions, and evidence was insufficient to determine if there was a difference (2 studies, 32 infants; very low-certainty evidence). There are conflicting data (5 studies) as to formula tolerance, with 4 studies reporting narrative results of no observed clinical difference and 1 study reporting higher incidence of signs of gastrointestinal intolerance in high MCT formula groups. There is no evidence of effect on the incidence of necrotizing enterocolitis (NEC), based on small numbers in two trials. Review authors found no studies addressing long-term growth parameters or neurodevelopmental outcomes.
Authors' conclusions: We found evidence of very low to low certainty suggesting no differences among short-term growth data for infants fed low versus high MCT formulas. Due to lack of evidence and uncertainty, neither formula type could be concluded to improve short-term growth outcomes or have fewer adverse effects. Further studies are necessary because the results from included studies are imprecise due to small numbers and do not address important long-term outcomes. Additional research should aim to clarify effects on formula tolerance and on long-term growth and neurodevelopmental outcomes, and should include larger study populations to better evaluate effect on NEC incidence.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
LJP has no interests to declare. HLB has no interests to declare. JIH has no interests to declare. LO works as a clinical dietitian specialist ‐ neonatal. She is a member of and a certified nutrition support clinician for the American Society for Parenteral and Enteral Nutrition (ASPEN).
Core editorial and administrative support for this review has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private foundation, independent from the Gerber Products Company. The grantor has no input on the content of the review nor the editorial process (see Sources of support.)
Figures








Update of
-
High versus low medium chain triglyceride content of formula for promoting short term growth of preterm infants.Cochrane Database Syst Rev. 2003;(1):CD002777. doi: 10.1002/14651858.CD002777. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2021 Feb 23;2:CD002777. doi: 10.1002/14651858.CD002777.pub2. PMID: 12535437 Updated.
Similar articles
-
High versus low medium chain triglyceride content of formula for promoting short term growth of preterm infants.Cochrane Database Syst Rev. 2003;(1):CD002777. doi: 10.1002/14651858.CD002777. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2021 Feb 23;2:CD002777. doi: 10.1002/14651858.CD002777.pub2. PMID: 12535437 Updated.
-
Individualized versus standard diet fortification for growth and development in preterm infants receiving human milk.Cochrane Database Syst Rev. 2020 Nov 23;11(11):CD013465. doi: 10.1002/14651858.CD013465.pub2. Cochrane Database Syst Rev. 2020. PMID: 33226632 Free PMC article.
-
High versus standard volume enteral feeds to promote growth in preterm or low birth weight infants.Cochrane Database Syst Rev. 2021 Mar 9;3(3):CD012413. doi: 10.1002/14651858.CD012413.pub3. Cochrane Database Syst Rev. 2021. PMID: 33733486 Free PMC article.
-
Continuous nasogastric milk feeding versus intermittent bolus milk feeding for preterm infants less than 1500 grams.Cochrane Database Syst Rev. 2021 Jun 24;6(6):CD001819. doi: 10.1002/14651858.CD001819.pub3. Cochrane Database Syst Rev. 2021. PMID: 34165778 Free PMC article.
-
Early fortification of human milk versus late fortification to promote growth in preterm infants.Cochrane Database Syst Rev. 2020 Jul 29;7(7):CD013392. doi: 10.1002/14651858.CD013392.pub2. Cochrane Database Syst Rev. 2020. PMID: 32726863 Free PMC article.
Cited by
-
Early nutrition and weight gain in preterm newborns and the risk of retinopathy of prematurity.PLoS One. 2013 May 29;8(5):e64325. doi: 10.1371/journal.pone.0064325. Print 2013. PLoS One. 2013. PMID: 23734194 Free PMC article.
-
Term infant formula macronutrient composition: An update for clinicians.J Pediatr Gastroenterol Nutr. 2025 May;80(5):751-759. doi: 10.1002/jpn3.70002. Epub 2025 Feb 10. J Pediatr Gastroenterol Nutr. 2025. PMID: 39930711 Free PMC article. Review.
-
The Role of Dietary Fats in the Development and Prevention of Necrotizing Enterocolitis.Nutrients. 2021 Dec 29;14(1):145. doi: 10.3390/nu14010145. Nutrients. 2021. PMID: 35011027 Free PMC article. Review.
-
Applications of Medium-Chain Triglycerides in Foods.Front Nutr. 2022 Jun 2;9:802805. doi: 10.3389/fnut.2022.802805. eCollection 2022. Front Nutr. 2022. PMID: 35719157 Free PMC article. Review.
-
Bifidobacterium longum Subspecies infantis Strain EVC001 Decreases Neonatal Murine Necrotizing Enterocolitis.Nutrients. 2022 Jan 24;14(3):495. doi: 10.3390/nu14030495. Nutrients. 2022. PMID: 35276854 Free PMC article.
References
References to studies included in this review
Armand 1996 {published data only}
Bustamante 1987 {published data only}
Carnielli 1996 {published data only}
-
- Carnielli VP, Rossi K, Badon T, Gregori B, Verlato G, Orzali A, et al. Medium-chain triacylglycerols in formulas for preterm infants: effect on plasma lipids, circulating concentrations of medium-chain fatty acids, and essential fatty acids. American Journal of Clinical Nutrition 1996;64(2):152-8. [DOI: 10.1093/ajcn/64.2.152] [PMID: ] - DOI - PubMed
Dutton 1987 {published data only}
Huston 1983 {published data only}
-
- Huston RK, Reynolds JW, Jensen C, Buist NR. Nutrient and mineral retention and vitamin D absorption in low-birth-weight infants: effect of medium-chain triglycerides. Pediatrics 1983;72(1):44-8. [PMID: ] - PubMed
Okamoto 1982 {published data only}
Rodriguez 2003 {published data only}
Sulkers 1992 {published data only}
-
- Sulkers EJ, Lafeber HN, Degenhart HJ, Lindemans J, Sauer PJ. Comparison of two preterm formulas with or without addition of medium-chain triglycerides (MCTs). II: effects on mineral balance. Journal of Pediatric Gastroenterology and Nutrition 1992;15(1):42-7. [DOI: 10.1097/00005176-199207000-00007] [PMID: ] - DOI - PubMed
-
- Sulkers EJ, Goudoever JB, Leunisse C, Wattimena JL, Sauer PJ. Comparison of two preterm formulas with or without addition of medium-chain triglycerides (MCTs). I: effects on nitrogen and fat balance and body composition changes. Journal of Pediatric Gastroenterology and Nutrition 1992;15(1):34-41. [DOI: 10.1097/00005176-199207000-00006] [PMID: ] - DOI - PubMed
Sulkers 1993a {published data only}
Wu 1993 {published data only}
-
- Wu PY, Edmond J, Morrow JW, Auestad N, Ponder D, Benson J. Gastrointestinal tolerance, fat absorption, plasma ketone and urinary dicarboxylic acid levels in low-birth-weight infants fed different amounts of medium-chain triglycerides in formula. Journal of Pediatric Gastroenterology and Nutrition 1993;17(2):145-52. [DOI: 10.1097/00005176-199308000-00004] [PMID: ] - DOI - PubMed
References to studies excluded from this review
Alliet 2007 {published data only}
Arsenault 2019 {published data only}
-
- Arsenault AB, Gunsalus KT, Laforce-Nesbitt SS, Przystac L, DeAngelis EJ, Hurley ME, et al. Dietary supplementation with medium-chain triglycerides reduces candida gastrointestinal colonization in preterm infants. Pediatric Infectious Disease Journal 2019;38(2):164-8. [DOI: 10.1097/INF.0000000000002042] [PMID: ] - DOI - PMC - PubMed
Axellson 1997 {published data only}
Bar‐Yoseph 2016 {published data only}
Boediman 1989 {published data only}
-
- Boediman D, Murakami R, Nakamura H, Matsuo T. Plasma apolipoprotein and lipid profiles in infants in the first year of life. Kobe Journal of Medical Sciences 1989;35(3):165-76. [PMID: ] - PubMed
Carnielli 1998 {published data only}
-
- Carnielli VP, Verlato G, Pederzini F, Luijendijk I, Boerlage A, Pedrotti D, et al. Intestinal absorption of long-chain polyunsaturated fatty acids in preterm infants fed breast milk or formula. American Journal of Clinical Nutrition 1998;67(1):97-103. [DOI: 10.1093/ajcn/67.1.97] [PMID: ] - DOI - PubMed
Faber 1988 {published data only}
Hamosh 1989 {published data only}
-
- Hamosh M, Bitman J, Liao T, Mehta NR, Buczek RJ, Wood DL, et al. Gastric lipolysis and fat absorption in preterm infants: effect of medium-chain triglyceride or long-chain triglyceride-containing formulas. Pediatrics 1989;83(1):86-92. [PMID: ] - PubMed
Hamosh 1991 {published data only}
-
- Hamosh M, Mehta NR, Fink CS, Coleman J, Hamosh P. Fat absorption in premature infants: medium-chain triglycerides and long-chain triglycerides are absorbed from formula at similar rates. Journal of Pediatric Gastroenterology and Nutrition 1991;13(2):143-9. [PMID: ] - PubMed
Hariharan 1995 {published data only}
Hayes 1992 {published data only}
-
- Hayes KC, Pronczuk A, Wood RA, Guy DG. Modulation of infant formula fat profile alters the low-density lipoprotein/high-density lipoprotein ratio and plasma fatty acid distribution relative to those with breast-feeding. Journal of Pediatrics 1992;120(4 Pt 2):S109-16. [DOI: 10.1016/s0022-3476(05)81244-2] [PMID: ] - DOI - PubMed
Hoffman 1992 {published data only}
Innis 2002 {published data only}
Lucas 1997 {published data only}
Mehes 1988 {published data only}
-
- Méhes K, Adamovich K. Effect of quality of feeding on serum lipoprotein levels in premature infants. Acta Paediatrica Hungarica 1988-1989;29(3-4):245-8. [PMID: ] - PubMed
O'Connor 2001 {published data only}
-
- O'Connor DL, Hall R, Adamkin D, Auestad N, Castillo M, Connor WE, et al, Ross Preterm Lipid Study. Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: a prospective, randomized controlled trial. Pediatrics 2001;108(2):359-71. [DOI: 10.1542/peds.108.2.359] [PMID: ] - DOI - PubMed
Pascale 1978 {published data only}
Ramirez 2001 {published data only}
-
- Ramírez M, Gallardo EM, Souto AS, Weissheimer C, Gil A. Plasma fatty-acid composition and antioxidant capacity in low birth-weight infants fed formula enriched with n-6 and n-3 long-chain polyunsaturated fatty acids from purified phospholipids. Clinical Nutrition 2001;20(1):69-76. [DOI: 10.1054/clnu.2000.0163] [PMID: ] - DOI - PubMed
Romera 2004 {published data only}
-
- Romera G, Figueras J, Rodriguez-Miguelez JM, Ortega J, Jimenez R. Energy intake, metabolic balance and growth in preterm infants fed formulas with different nonprotein energy supplements. Journal of Pediatric Gastroenterology and Nutrition 2004;38(4):407-13. [DOI: 10.1097/00005176-200404000-00008] [PMID: ] - DOI - PubMed
Roy 1975 {published data only}
Siahanidou 2008 {published data only}
-
- Siahanidou T, Margeli A, Lazaropoulou C, Karavitakis E, Papassotiriou I, Mandyla H. Circulating adiponectin in preterm infants fed long-chain polyunsaturated fatty acids (LCPUFA)-supplemented formula - a randomized controlled study. Pediatric Research 2008;63(4):428-32. [DOI: 10.1203/PDR.0b013e31816780e4] [PMID: ] - DOI - PubMed
Siahanidou 2011 {published data only}
-
- Siahanidou T, Margeli A, Kappis A, Papassotiriou I, Mandyla H. Circulating visfatin levels in healthy preterm infants are independently associated with high-density lipoprotein cholesterol levels and dietary long-chain polyunsaturated fatty acids. Metabolism. 2011;60(3):389-93. [DOI: 10.1016/j.metabol.2010.03.002] [PMID: ] - DOI - PubMed
Sidebottom 1983 {published data only}
Smith 1988 {published data only}
Spencer 1986 {published data only}
Spencer 1992 {published data only}
Sutphen 1992 {published data only}
Tantibhedhyangkul 1975 {published data only}
-
- Tantibhedhyangkul P, Hashim SA. Medium-chain triglyceride feeding in premature infants: effects on fat and nitrogen absorption. Pediatrics 1975;55(3):359-70. [PMID: ] - PubMed
Tantibhedhyangkul 1978 {published data only}
-
- Tantibhedhyangkul P, Hashim SA. Medium-chain triglyceride feeding in premature infants: effects on calcium and magnesium absorption. Pediatrics 1978;61(4):537-45. [PMID: ] - PubMed
Telliez 1998 {published data only}
Telliez 2002 {published data only}
-
- Telliez F, Bach V, Leke A, Chardon K, Libert JP. Feeding behavior in neonates whose diet contained medium-chain triacylglycerols: short-term effects on thermoregulation and sleep. American Journal of Clinical Nutrition 2002;76(5):1091-5. [DOI: 10.1093/ajcn/76.5.1091] [PMID: ] - PubMed
Thanh 2018 {published data only}
-
- Thanh LQ, Chen YM, Hartweg M, Nguyen T, Tu A. Effects of higher protein formula with improved fat blend on growth and feeding tolerance in preterm Infants: a double-blind, randomized, controlled clinical trial. In: Excellence in Pediatrics, Oral Presentation. 2018 December 6-8. 2018. [Abstract ID 255]
Van Aerde 1985 {published data only}
-
- Van Aerde J, Sauer P, Heim T, Smith J, Swyer P. The effect of diet composition on energy metabolism, substrate utilisation and growth in the VLBW infant (abstract). Pediatric Research 1985;19:1085. [DOI: 10.1203/00006450-198510000-00104] - DOI
Vanderhoof 1999 {published data only}
-
- Vanderhoof J, Gross S, Hegyi T, Clandinin T, Porcelli P, DeCristofaro J, et al. Evaluation of a long-chain polyunsaturated fatty acid supplemented formula on growth, tolerance, and plasma lipids in preterm infants up to 48 weeks postconceptional age. Journal of Pediatric Gastroenterology and Nutrition 1999;29(3):318-26. [DOI: 10.1097/00005176-199909000-00015] [PMID: ] - DOI - PubMed
Verkade 1989 {published data only}
Whyte 1986 {published data only}
-
- Whyte RK, Campbell D, Stanhope R, et al. Energy balance in low birth weight infants fed formula of high or low medium-chain triglyceride content. Journal of Pediatrics 1986;108(6):964-71. [DOI: 10.1016/s0022-3476(86)80941-6] [PMID: ] - PubMed
Additional references
Ehrenkranz 1999
Fenton 2013
Fleith 2005
-
- Fleith M, Clandinin MT. Dietary PUFA for preterm and term infants: review of clinical studies. Critical Reviews in Food Science and Nutrition 2005;45(3):205-29. [DOI: 10.1080/10408690590956378] [PMID: ] - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), June 5, 2020. Available at gradepro.org.
Hanson 2011
Heird 2005
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Jensen 1992
Kleinman 2019
-
- Kleinman R, Greer F. Pediatric Nutrition, 8th edition. Chicago, IL: American Academy of Pediatrics Committee on Nutrition, 2019. [ISBN: 978-1-61002-360-3]
Lee 2015
-
- Lee KA, Hayes BC. Head size and growth in the very preterm infant: a literature review. Research and Reports in Neonatology 2015;5:1–7. [DOI: 10.2147/RRN.S74449] - DOI
Los‐Rycharska 2016
Marten 2006
-
- Marten B, Pfeuffer M. Medium-chain triglycerides. International Dairy Journal 2006;16(11):1374-82.
Mosca 2017
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Cochrane Collaboration, 2020.
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sodhi 2018
Traul 2000
Vinall 2013
Walsh 1986
Wright 2006
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

