Does biomarker use in oncology improve clinical trial failure risk? A large-scale analysis
- PMID: 33620160
- PMCID: PMC7957156
- DOI: 10.1002/cam4.3732
Does biomarker use in oncology improve clinical trial failure risk? A large-scale analysis
Abstract
Purpose: To date there has not been an extensive analysis of the outcomes of biomarker use in oncology.
Methods: Data were pooled across four indications in oncology drawing upon trial outcomes from www.clinicaltrials.gov: breast cancer, non-small cell lung cancer (NSCLC), melanoma and colorectal cancer from 1998 to 2017. We compared the likelihood drugs would progress through the stages of clinical trial testing to approval based on biomarker status. This was done with multi-state Markov models, tools that describe the stochastic process in which subjects move among a finite number of states.
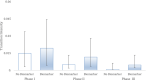
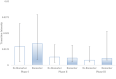
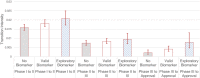
Results: Over 10000 trials were screened, which yielded 745 drugs. The inclusion of biomarker status as a covariate significantly improved the fit of the Markov model in describing the drug trajectories through clinical trial testing stages. Hazard ratios based on the Markov models revealed the likelihood of drug approval with biomarkers having nearly a fivefold increase for all indications combined. A 12, 8 and 7-fold hazard ratio was observed for breast cancer, melanoma and NSCLC, respectively. Markov models with exploratory biomarkers outperformed Markov models with no biomarkers.
Conclusion: This is the first systematic statistical evidence that biomarkers clearly increase clinical trial success rates in three different indications in oncology. Also, exploratory biomarkers, long before they are properly validated, appear to improve success rates in oncology. This supports early and aggressive adoption of biomarkers in oncology clinical trials.
Keywords: biomarkers; breast cancer; cancer; clinical trial; drug development; lung cancer; melanoma; oncology; risk.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Parker has worked in the pharmaceutical industry. Dr. Lopes has received honoraria, has had a consultant role and has received research funding from Pfizer, Merck Serono, Roche, AstraZeneca and Eli Lilly in the past.
Figures



References
-
- American Cancer Society , Cancer Facts & Figures. 2018. Available from https://www.cancer.org/content/dam/cancer‐org/research/cancer‐facts‐and‐....
-
- Falconi A, Lopes G, Parker JL. Biomarker and receptor targeted therapies reduce clinical trial risk in non‐small cell lung cancer. J Thorac Oncol. 2014;9(2):163‐169. - PubMed
-
- Rubinger DA, Hollmann SS, Serdetchnaia V, Ernst DS, Parker JL. Biomarker use is associated with reduced clinical trial failure risk in metastatic melanoma. Biomark Med. 2015;9(1):13‐23. - PubMed
-
- Parker JL, Zhang ZY, Buckstein R. Clinical trial risk in Non‐Hodgkin's lymphoma: endpoint and target selection. J Pharm Pharm Sci. 2011;14(2):227‐235. - PubMed
-
- Tenuta J, Klotz L, Parker JL. Clinical trial risk in castration resistant prostate cancer: immunotherapies show promise. BJU Int. 2014;113(5b):E82‐E89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

