Effectiveness of Internet-Based Exercises Aimed at Treating Knee Osteoarthritis: The iBEAT-OA Randomized Clinical Trial
- PMID: 33620447
- PMCID: PMC7903254
- DOI: 10.1001/jamanetworkopen.2021.0012
Effectiveness of Internet-Based Exercises Aimed at Treating Knee Osteoarthritis: The iBEAT-OA Randomized Clinical Trial
Erratum in
-
Error in Visual Abstract.JAMA Netw Open. 2021 Mar 1;4(3):e216209. doi: 10.1001/jamanetworkopen.2021.6209. JAMA Netw Open. 2021. PMID: 33764417 Free PMC article. No abstract available.
Abstract
Importance: Osteoarthritis is a prevalent, debilitating, and costly chronic disease for which recommended first-line treatment is underused.
Objective: To compare the effect of an internet-based treatment for knee osteoarthritis vs routine self-management (ie, usual care).
Design, setting, and participants: This randomized clinical trial was conducted from October 2018 to March 2020. Participants included individuals aged 45 years or older with a diagnosis of knee osteoarthritis recruited from an existing primary care database or from social media advertisements were invited. Data were analyzed April to July 2020.
Interventions: The intervention and control group conformed to first-line knee osteoarthritis treatment. For the intervention group, treatment was delivered via a smartphone application. The control group received routine self-management care.
Main outcomes and measures: The primary outcome was change from baseline to 6 weeks in self-reported pain during the last 7 days, reported on a numerical rating scale (NRS; range, 0-10, with 0 indicating no pain and 10, worst pain imaginable), compared between groups. Secondary outcomes included 2 physical functioning scores, hamstring and quadriceps muscle strength, the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), and quantitative sensory testing.
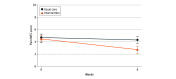
Results: Among a total of 551 participants screened for eligibility, 146 were randomized and 105 were analyzed (mean [SD] age, 66.7 [9.2] years, 71 [67.1%] women), including 48 participants in the intervention group and 57 participants in the control group. There were no significant differences in baseline characteristics between the groups. At the 6-week follow-up, the intervention group showed a greater NRS pain score reduction than the control group (between-group difference, -1.5 [95% CI, -2.2 to -0.8]; P < .001). Similarly, the intervention group had better improvements in the 30-second sit-to-stand test (between-group difference, 3.4 [95% CI, 2.2 to 4.5]; P < .001) and Timed Up-and-Go test (between-group difference, -1.8 [95% CI, -3.0 to -0.5] seconds; P = .007), as well as the WOMAC subscales for pain (between-group difference, -1.1 [95% CI, -2.0 to -0.2]; P = .02), stiffness (between-group difference, -1.0 [95% CI, -1.5 to -0.5]; P < .001), and physical function (between-group difference, -3.4 [95% CI, -6.2 to -0.7]; P = .02). The magnitude of within-group changes in pain (d = 0.83) and function outcomes (30 second sit-to-stand test d = 1.24; Timed Up-and-Go test d = 0.76) in the intervention group corresponded to medium to very strong effects. No adverse events were reported.
Conclusions and relevance: These findings suggest that this internet-delivered, evidence-based, first-line osteoarthritis treatment was superior to routine self-managed usual care and could be provided without harm to people with osteoarthritis. Effect sizes observed in the intervention group corresponded to clinically important improvements.
Trial registration: ClinicalTrials.gov Identifier: NCT03545048.
Conflict of interest statement
Figures


References
-
- McCormick A, Fleming D, Charlton J. Morbidity Statistics From General Practice: Fourth National Study 1991-1992. HMSO; 1995.
-
- Conaghan PG, Dickson J, Grant RL; Guideline Development Group . Care and management of osteoarthritis in adults: summary of NICE guidance. BMJ. 2008;336(7642):502-503. doi: 10.1136/bmj.39490.608009.AD - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

