Analysis of formin functions during cytokinesis using specific inhibitor SMIFH2
- PMID: 33620500
- PMCID: PMC8195507
- DOI: 10.1093/plphys/kiab085
Analysis of formin functions during cytokinesis using specific inhibitor SMIFH2
Abstract
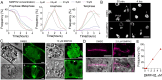
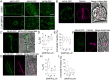
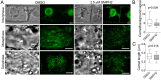
The phragmoplast separates daughter cells during cytokinesis by constructing the cell plate, which depends on interaction between cytoskeleton and membrane compartments. Proteins responsible for these interactions remain unknown, but formins can link cytoskeleton with membranes and several members of formin protein family localize to the cell plate. Progress in functional characterization of formins in cytokinesis is hindered by functional redundancies within the large formin gene family. We addressed this limitation by employing Small Molecular Inhibitor of Formin Homology 2 (SMIFH2), a small-molecule inhibitor of formins. Treatment of tobacco (Nicotiana tabacum) tissue culture cells with SMIFH2 perturbed localization of actin at the cell plate; slowed down both microtubule polymerization and phragmoplast expansion; diminished association of dynamin-related proteins with the cell plate independently of actin and microtubules; and caused cell plate swelling. Another impact of SMIFH2 was shortening of the END BINDING1b (EB1b) and EB1c comets on the growing microtubule plus ends in N. tabacum tissue culture cells and Arabidopsis thaliana cotyledon epidermis cells. The shape of the EB1 comets in the SMIFH2-treated cells resembled that of the knockdown mutant of plant Xenopus Microtubule-Associated protein of 215 kDa (XMAP215) homolog MICROTUBULE ORGANIZATION 1/GEMINI 1 (MOR1/GEM1). This outcome suggests that formins promote elongation of tubulin flares on the growing plus ends. Formins AtFH1 (A. thaliana Formin Homology 1) and AtFH8 can also interact with EB1. Besides cytokinesis, formins function in the mitotic spindle assembly and metaphase to anaphase transition. Our data suggest that during cytokinesis formins function in: (1) promoting microtubule polymerization; (2) nucleating F-actin at the cell plate; (3) retaining dynamin-related proteins at the cell plate; and (4) remodeling of the cell plate membrane.
© American Society of Plant Biologists 2021. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures






Comment in
-
Formins bridle and unleash their way through cytokinesis: a phragmoplast-centered view.Plant Physiol. 2021 Jun 11;186(2):829-831. doi: 10.1093/plphys/kiab148. Plant Physiol. 2021. PMID: 33842968 Free PMC article. No abstract available.
References
-
- Akhmanova A, Steinmetz MO (2008) Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol 9: 309–322 - PubMed
-
- Asada T, Sonobe S, Shibaoka H (1991) Microtubule translocation in the cytokinetic apparatus of cultured tobacco cells. Nature 350: 238–241
-
- Austin JR II, Segui-Simarro JM, Staehelin LA (2005) Quantitative analysis of changes in spatial distribution and plus-end geometry of microtubules involved in plant-cell cytokinesis. J Cell Sci 118: 3895–3903 - PubMed
-
- Bajer A (1968) Fine structure studies on phragmoplast and cell plate formation. Chromosoma 24: 383–417
-
- Baluska F, Jasik J, Edelmann HG, Salajova T, Volkmann D (2001) Latrunculin B-induced plant dwarfism: Plant cell elongation is F-actin-dependent. Dev Biol 231: 113–124 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

