Evidence-based clinical practice guidelines for peptic ulcer disease 2020
- PMID: 33620586
- PMCID: PMC8005399
- DOI: 10.1007/s00535-021-01769-0
Evidence-based clinical practice guidelines for peptic ulcer disease 2020
Abstract
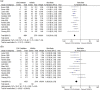
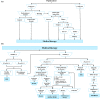
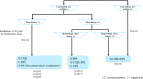
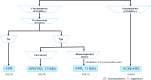
The Japanese Society of Gastroenterology (JSGE) revised the third edition of evidence-based clinical practice guidelines for peptic ulcer disease in 2020 and created an English version. The revised guidelines consist of nine items: epidemiology, hemorrhagic gastric and duodenal ulcers, Helicobacter pylori (H. pylori) eradication therapy, non-eradication therapy, drug-induced ulcers, non-H. pylori, and nonsteroidal anti-inflammatory drug (NSAID) ulcers, remnant gastric ulcers, surgical treatment, and conservative therapy for perforation and stenosis. Therapeutic algorithms for the treatment of peptic ulcers differ based on ulcer complications. In patients with NSAID-induced ulcers, NSAIDs are discontinued and anti-ulcer therapy is administered. If NSAIDs cannot be discontinued, the ulcer is treated with proton pump inhibitors (PPIs). Vonoprazan (VPZ) with antibiotics is recommended as the first-line treatment for H. pylori eradication, and PPIs or VPZ with antibiotics is recommended as a second-line therapy. Patients who do not use NSAIDs and are H. pylori negative are considered to have idiopathic peptic ulcers. Algorithms for the prevention of NSAID- and low-dose aspirin (LDA)-related ulcers are presented in this guideline. These algorithms differ based on the concomitant use of LDA or NSAIDs and ulcer history or hemorrhagic ulcer history. In patients with a history of ulcers receiving NSAID therapy, PPIs with or without celecoxib are recommended and the administration of VPZ is suggested for the prevention of ulcer recurrence. In patients with a history of ulcers receiving LDA therapy, PPIs or VPZ are recommended and the administration of a histamine 2-receptor antagonist is suggested for the prevention of ulcer recurrence.
Keywords: Helicobacter pylori eradication; Idiopathic ulcer; Low-dose aspirin; Nonsteroidal anti-inflammatory drug; Peptic ulcer.
Conflict of interest statement
Any financial relationship with enterprises, businesses or academic institutions in the subject matter or materials discussed in the manuscript are listed as follows: (1) those from whom the authors, the spouse, partner or immediate relatives of authors, who have received individually any income, honoraria or any other types of remuneration: Astellas Pharma Inc., AstraZeneca K.K., DaiichiSankyo Company, Limited, Eisai Co., Ltd., Otsuka Pharmaceutical Co.,Ltd., Pfizer Japan Inc., Takeda Pharmaceutical Company Limited. and (2) those from whom the academic institutions of the authors received support (commercial/academic cooperation): Ajinomoto Pharmaceuticals Co., Ltd., AsTellas Pharma Inc., AstraZenecaK. K., Bayer Yakuhin, Ltd., Chugai PharmaCeutical Co., Ltd., DaiichiSankyo Company, Limited, Eisai Co., Ltd., Kishuhosokawa Co., Ltd., Maruso Co., Ltd, Mitsubishi Tanabe Pharma Corporation, MSD K.K., Nihon Pharmaceutical Co. Ltd., Nippon Shinyaku Co., Ltd., Okahatanoen Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Sanofi K.K., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical.
Figures
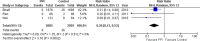
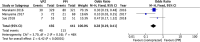
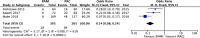

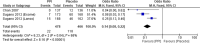
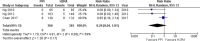

References
-
- Yoshida M, Kinoshita Y, Watanabe M, et al. JSGE clinical practice guideline 2014: standards, methods, and process of developing guidelines. J Gastroenterol. 2015;50:4–10. - PubMed
-
- Fujimoto K, Fujishiro M, Kato M, et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc. 2014;26:1–14. - PubMed
-
- Kato M, Uedo N, Hokimoto S, et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment: 2017 appendix on anticoagulants including direct oral anticoagulants. Dig Endosc. 2018;30:433–440. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

