Inclisiran: First Approval
- PMID: 33620677
- PMCID: PMC7900795
- DOI: 10.1007/s40265-021-01473-6
Inclisiran: First Approval
Erratum in
-
Correction to: Inclisiran: First Approval.Drugs. 2021 Jun;81(9):1129. doi: 10.1007/s40265-021-01529-7. Drugs. 2021. PMID: 33983616 Free PMC article. No abstract available.
Abstract
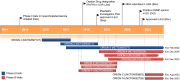
Inclisiran (Leqvio®; Novartis) is a first-in-class, cholesterol-lowering small interfering RNA (siRNA) conjugated to triantennary N-acetylgalactosamine carbohydrates (GalNAc). Inclisiran received its first approval in December 2020 in the EU for use in adults with primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia, as an adjunct to diet. It is intended for use in combination with a statin or a statin with other lipid-lowering therapies in patients unable to reach low-density lipoprotein cholesterol goals with the maximum tolerated statin dose. In patients who are statin-intolerant or for whom a statin is contraindicated, inclisiran can be used alone or in combination with other lipid-lowering therapies. Inclisiran is administered as a twice-yearly subcutaneous injection. This article summarizes the milestones in the development of inclisiran leading to this first approval for primary hypercholesterolaemia or mixed dyslipidaemia.
Conflict of interest statement
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Yvette Lamb is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Figures
References
-
- European Medicines Agency. Leqvio 284 mg solution for injection in pre-filled syringe: summary of product characteristics. 2021. http://www.ema.europa.eu/. Accessed 25 Jan 2021.
-
- European Medicines Agency. Leqvio (inclisiran): an overview of Leqvio and why it is authorised in the EU. 2021. http://www.ema.europa.eu/. Accessed 25 Jan 2021.
-
- Novartis. Novartis receives EU approval for Leqvio® (inclisiran), a first-in-class siRNA to lower cholesterol with two doses a year [media release]. 11 Dec 2020. http://www.novartis.com/.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical