Cost-effectiveness of hepatitis C virus (HCV) elimination strategies among people who inject drugs (PWID) in Tijuana, Mexico
- PMID: 33620750
- PMCID: PMC8380744
- DOI: 10.1111/add.15456
Cost-effectiveness of hepatitis C virus (HCV) elimination strategies among people who inject drugs (PWID) in Tijuana, Mexico
Abstract
Background and aims: In Latin America, Mexico was first to launch a hepatitis C virus (HCV) elimination strategy, where people who inject drugs (PWID) are a main risk group for transmission. In Tijuana, HCV seroprevalence among PWID is > 90%, with minimal harm reduction (HR). We evaluated cost-effectiveness of strategies to achieve the incidence elimination target among PWID in Tijuana.
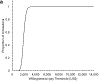
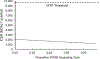
Methods: Modeling study using a dynamic, cost-effectiveness model of HCV transmission and progression among active and former PWID in Tijuana, to assess the cost-effectiveness of incidence elimination strategies from a health-care provider perspective. The model incorporated PWID transitions between HR stages (no HR, only opioid agonist therapy, only high coverage needle-syringe programs, both). Four strategies that could achieve the incidence target (80% reduction by 2030) were compared with the status quo (no intervention). The strategies incorporated the number of direct-acting anti-viral (DAA) treatments required with: (1) no HR scale-up, (2) HR scale-up from 2019 to 20% coverage among PWID, (3) HR to 40% coverage and (4) HR to 50% coverage. Costs (2019 US$) and health outcomes [disability-adjusted life years (DALYs)] were discounted 3% per year. Mean incremental cost-effectiveness ratios (ICER, $/DALY averted) were compared with one-time per capita gross domestic product (GDP) ($9698 in 2019) and purchasing power parity-adjusted per capita GDP ($4842-13 557) willingness-to-pay (WTP) thresholds.
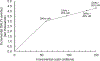
Results: DAAs alone were the least costly elimination strategy [$173 million, 95% confidence interval (CI) = 126-238 million], but accrued fewer health benefits compared with strategies with HR. DAAs + 50% HR coverage among PWID averted the most DALYs but cost $265 million, 95% CI = 210-335 million). The optimal strategy was DAAs + 50% HR (ICER $6743/DALY averted compared to DAAs only) under the one-time per-capita GDP WTP ($9698).
Conclusions: A combination of high-coverage harm reduction and hepatitis C virus treatment is the optimal cost-effective strategy to achieve the HCV incidence elimination goal in Mexico.
Keywords: Cost-effectiveness; HCV; harm reduction; modeling; people who inject drugs; prevention.
© 2021 Society for the Study of Addiction.
Figures

Similar articles
-
Is hepatitis C virus (HCV) elimination achievable among people who inject drugs in Tijuana, Mexico? A modeling analysis.Int J Drug Policy. 2021 Feb;88:102710. doi: 10.1016/j.drugpo.2020.102710. Epub 2020 Mar 9. Int J Drug Policy. 2021. PMID: 32165050 Free PMC article.
-
An intensive model of care for hepatitis C virus screening and treatment with direct-acting antivirals in people who inject drugs in Nairobi, Kenya: a model-based cost-effectiveness analysis.Addiction. 2022 Feb;117(2):411-424. doi: 10.1111/add.15630. Epub 2021 Jul 28. Addiction. 2022. PMID: 34184794 Free PMC article.
-
Effectiveness and cost-effectiveness of interventions targeting harm reduction and chronic hepatitis C cascade of care in people who inject drugs: The case of France.J Viral Hepat. 2018 Oct;25(10):1197-1207. doi: 10.1111/jvh.12919. Epub 2018 May 9. J Viral Hepat. 2018. PMID: 29660211
-
Is hepatitis C virus elimination possible among people living with HIV and what will it take to achieve it?J Int AIDS Soc. 2018 Apr;21 Suppl 2(Suppl Suppl 2):e25062. doi: 10.1002/jia2.25062. J Int AIDS Soc. 2018. PMID: 29633560 Free PMC article. Review.
-
Hepatitis C virus micro-elimination in people who inject drugs: Challenges and chance in Taiwan and worldwide.Kaohsiung J Med Sci. 2024 Feb;40(2):112-118. doi: 10.1002/kjm2.12788. Epub 2023 Nov 27. Kaohsiung J Med Sci. 2024. PMID: 38010851 Free PMC article. Review.
Cited by
-
Hepatitis C elimination among people who inject drugs in Mexico during the COVID-19 pandemic.Gac Med Mex. 2022;158(2):110-113. doi: 10.24875/GMM.M22000650. Gac Med Mex. 2022. PMID: 35763823 Free PMC article.
-
Cost-effectiveness of a police education program on HIV and overdose among people who inject drugs in Tijuana, Mexico.Lancet Reg Health Am. 2024 Feb 1;30:100679. doi: 10.1016/j.lana.2024.100679. eCollection 2024 Feb. Lancet Reg Health Am. 2024. PMID: 38327278 Free PMC article.
-
Health and Economic Impact of Periodic Hepatitis C Virus Testing Among People Who Inject Drugs.JAMA Health Forum. 2025 Jul 3;6(7):e251870. doi: 10.1001/jamahealthforum.2025.1870. JAMA Health Forum. 2025. PMID: 40608305 Free PMC article.
-
Economic evaluation of the Hepatitis C virus elimination program in the country of Georgia, 2015 to 2017.Liver Int. 2023 Mar;43(3):558-568. doi: 10.1111/liv.15431. Epub 2022 Oct 4. Liver Int. 2023. PMID: 36129625 Free PMC article.
-
Modelling the contribution of incarceration and public health oriented drug law reform to HCV transmission and elimination among PWID in Tijuana, Mexico.Int J Drug Policy. 2022 Dec;110:103878. doi: 10.1016/j.drugpo.2022.103878. Epub 2022 Oct 12. Int J Drug Policy. 2022. PMID: 36242829 Free PMC article.
References
-
- Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, et al.Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. The Lancet Global health. 2017;5(12):e1192–e207. - PMC - PubMed
-
- Polaris Observatory HCVC. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–76. - PubMed
-
- Graham CS, Swan T. A path to eradication of hepatitis C in low- and middle-income countries. Antiviral Research. 2015;119:89–96. - PubMed
-
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology (Baltimore, Md). 2013;57(4):1333–42. - PubMed
-
- Platt L, Minozzi S, Reed J, Vickerman P, Hagan H, French C, et al.Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction (Abingdon, England). 2018;113(3):545–63. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

