ROBUST: A Phase III Study of Lenalidomide Plus R-CHOP Versus Placebo Plus R-CHOP in Previously Untreated Patients With ABC-Type Diffuse Large B-Cell Lymphoma
- PMID: 33621109
- PMCID: PMC8078325
- DOI: 10.1200/JCO.20.01366
ROBUST: A Phase III Study of Lenalidomide Plus R-CHOP Versus Placebo Plus R-CHOP in Previously Untreated Patients With ABC-Type Diffuse Large B-Cell Lymphoma
Abstract
Purpose: Patients with the activated B-cell-like (ABC) subtype of diffuse large B-cell lymphoma (DLBCL) historically showed inferior survival with standard rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). Phase II studies demonstrated that adding the immunomodulatory agent lenalidomide to R-CHOP improved outcomes in ABC-type DLBCL. The goal of the global, phase III ROBUST study was to compare lenalidomide plus R-CHOP (R2-CHOP) with placebo/R-CHOP in previously untreated, ABC-type DLBCL.
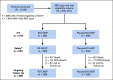
Methods: Histology and cell-of-origin type were prospectively analyzed by central pathology prior to random assignment and study treatment. Patients with ABC-DLBCL received lenalidomide oral 15 mg/d, days 1-14/21 plus standard R-CHOP21 versus placebo/R-CHOP21 for six cycles. The primary end point was progression-free survival (PFS) per independent central radiology review.
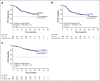
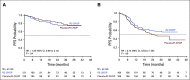
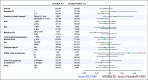
Results: A total of 570 patients with ABC-DLBCL (n = 285 per arm) were stratified by International Prognostic Index score, age, and bulky disease, and randomly assigned to R2-CHOP or placebo/R-CHOP. Baseline demographics were similar between arms. Most patients completed six cycles of treatment: 74% R2-CHOP and 84% placebo/R-CHOP. The most common grade 3/4 adverse events for R2-CHOP versus placebo/R-CHOP were neutropenia (60% v 48%), anemia (22% v 14%), thrombocytopenia (17% v 11%), and leukopenia (14% v 15%). The primary end point of PFS was not met, with a hazard ratio of 0.85 (95% CI, 0.63 to 1.14) and P = .29; median PFS has not been reached for either arm. PFS trends favoring R2-CHOP over placebo/R-CHOP were seen in patients with higher-risk disease.
Conclusion: ROBUST is the first DLBCL phase III study to integrate biomarker-driven identification of eligible ABC patients. Although the ROBUST trial did not meet the primary end point of PFS in all patients, the safety profile of R2-CHOP was consistent with individual treatments with no new safety signals.
Trial registration: ClinicalTrials.gov NCT02285062.
Conflict of interest statement
Figures

Comment in
-
Precision Medicine in DLBCL: Are We There Yet?J Clin Oncol. 2021 Apr 20;39(12):1314-1316. doi: 10.1200/JCO.20.03531. Epub 2021 Feb 23. J Clin Oncol. 2021. PMID: 33621123 No abstract available.
References
-
- Armitage JO, Weisenburger DD: New approach to classifying non-Hodgkin's lymphomas: Clinical features of the major histologic subtypes. Non-Hodgkin's lymphoma classification project. J Clin Oncol 16:2780-2795, 1998 - PubMed
-
- Swerdlow SH Campo E Harris NL, et al. : WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France, International Agency for Research on Cancer, 2008
-
- Coiffier B Lepage E Briere J, et al. : CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 346:235-242, 2002 - PubMed
-
- Habermann TM Weller EA Morrison VA, et al. : Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol 24:3121-3127, 2006 - PubMed
-
- Tilly H Gomes da Silva M Vitolo U, et al. : Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 26:v116-v125, 2015. (suppl 5) - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

