Digital Health Integration Assessment and Maturity of the United States Biopharmaceutical Industry: Forces Driving the Next Generation of Connected Autoinjectable Devices
- PMID: 33621188
- PMCID: PMC8088878
- DOI: 10.2196/25406
Digital Health Integration Assessment and Maturity of the United States Biopharmaceutical Industry: Forces Driving the Next Generation of Connected Autoinjectable Devices
Abstract
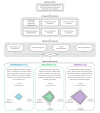
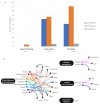
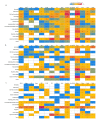
Autoinjectable devices continue to provide real-life benefits for patients with chronic conditions since their widespread adoption 30 years ago with the rise of macromolecules. Nonetheless, issues surrounding adherence, patient administration techniques, disease self-management, and data outcomes at scale persist despite product design innovation. The interface of drug device combination products and digital health technologies formulates a value proposition for next-generation autoinjectable devices to power the delivery of precision care at home and achieve the full potential of biologics. Success will largely be dependent on biopharma's digital health maturity to implement this framework. This viewpoint measures the digital health maturity of the top 15 biopharmaceutical companies in the US biologics autoinjector market and establishes the framework for next-generation autoinjectable devices powering home-based precision care and the need for formal digital health training.
Keywords: artificial intelligence; autoimmune; autoinjector; biopharma; digital health; disease management; drug delivery; injectable devices; oncology; rare diseases.
©Ramin Rafiei, Chelsea Williams, Jeannette Jiang, Timothy Dy Aungst, Matthias Durrer, Dao Tran, Ralph Howald. Originally published in JMIR mHealth and uHealth (http://mhealth.jmir.org), 18.03.2021.
Conflict of interest statement
Conflicts of Interest: TDA has served as a consultant or advisor for Otsuka Pharmaceuticals, Teva, and Eli Lilly. He is on Otsuka Pharmaceuticals speaker’s bureau. He is also an advisor for HealthXL and The Digital Therapeutics Alliance. The other authors declare no competing interests.
Figures

Similar articles
-
Clinical Integration of Digital Solutions in Health Care: An Overview of the Current Landscape of Digital Technologies in Cancer Care.JCO Clin Cancer Inform. 2018 Dec;2:1-9. doi: 10.1200/CCI.17.00159. JCO Clin Cancer Inform. 2018. PMID: 30652580 Review.
-
The changing environment for technological innovation in health care.Baxter Health Policy Rev. 1996;2:267-315. Baxter Health Policy Rev. 1996. PMID: 11066263
-
Improving Digital Hospital Transformation: Development of an Outcomes-Based Infrastructure Maturity Assessment Framework.JMIR Med Inform. 2019 Jan 11;7(1):e12465. doi: 10.2196/12465. JMIR Med Inform. 2019. PMID: 30632973 Free PMC article.
-
The Significance of Alliance Networks in Research and Development of Digital Health Products for Diabetes: Observational Study.JMIR Diabetes. 2021 Oct 21;6(4):e32446. doi: 10.2196/32446. JMIR Diabetes. 2021. PMID: 34673525 Free PMC article.
-
Design control considerations for biologic-device combination products.Adv Drug Deliv Rev. 2017 Mar;112:101-105. doi: 10.1016/j.addr.2017.01.003. Epub 2017 Jan 11. Adv Drug Deliv Rev. 2017. PMID: 28088344 Review.
Cited by
-
India's Opportunity to Address Human Resource Challenges in Healthcare.Cureus. 2023 Jun 11;15(6):e40274. doi: 10.7759/cureus.40274. eCollection 2023 Jun. Cureus. 2023. PMID: 37448434 Free PMC article. Review.
-
Digital Therapeutics for Improving Effectiveness of Pharmaceutical Drugs and Biological Products: Preclinical and Clinical Studies Supporting Development of Drug + Digital Combination Therapies for Chronic Diseases.J Clin Med. 2024 Jan 11;13(2):403. doi: 10.3390/jcm13020403. J Clin Med. 2024. PMID: 38256537 Free PMC article. Review.
-
Risks and benefits associated with the primary functions of artificial intelligence powered autoinjectors.Front Med Technol. 2024 Apr 5;6:1331058. doi: 10.3389/fmedt.2024.1331058. eCollection 2024. Front Med Technol. 2024. PMID: 38645777 Free PMC article.
-
Digital health and capability maturity models-a critical thematic review and conceptual synthesis of the literature.J Am Med Inform Assoc. 2023 Jan 18;30(2):393-406. doi: 10.1093/jamia/ocac228. J Am Med Inform Assoc. 2023. PMID: 36451257 Free PMC article. Review.
-
Redesigning Pharmacy to Improve Public Health Outcomes: Expanding Retail Spaces for Digital Therapeutics to Replace Consumer Products That Increase Mortality and Morbidity Risks.Pharmacy (Basel). 2024 Jul 12;12(4):107. doi: 10.3390/pharmacy12040107. Pharmacy (Basel). 2024. PMID: 39051391 Free PMC article.
References
-
- Snowden S. Digital Health: A Framework for Healthcare Transformation. Healthcare Information and Management System Society. 2020. [2020-09-21]. https://www.himss.org/resources/digital-health-framework-healthcare-tran....
-
- What is Digital Health? FDA. [2020-09-21]. https://www.fda.gov/medical-devices/digital-health-center-excellence/wha....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

