Cx43 hemichannel microdomain signaling at the intercalated disc enhances cardiac excitability
- PMID: 33621213
- PMCID: PMC8011902
- DOI: 10.1172/JCI137752
Cx43 hemichannel microdomain signaling at the intercalated disc enhances cardiac excitability
Abstract
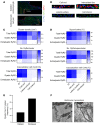
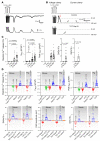
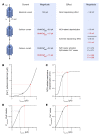
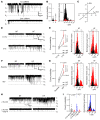
Cx43, a major cardiac connexin, forms precursor hemichannels that accrue at the intercalated disc to assemble as gap junctions. While gap junctions are crucial for electrical conduction in the heart, little is known about the potential roles of hemichannels. Recent evidence suggests that inhibiting Cx43 hemichannel opening with Gap19 has antiarrhythmic effects. Here, we used multiple electrophysiology, imaging, and super-resolution techniques to understand and define the conditions underlying Cx43 hemichannel activation in ventricular cardiomyocytes, their contribution to diastolic Ca2+ release from the sarcoplasmic reticulum, and their impact on electrical stability. We showed that Cx43 hemichannels were activated during diastolic Ca2+ release in single ventricular cardiomyocytes and cardiomyocyte cell pairs from mice and pigs. This activation involved Cx43 hemichannel Ca2+ entry and coupling to Ca2+ release microdomains at the intercalated disc, resulting in enhanced Ca2+ dynamics. Hemichannel opening furthermore contributed to delayed afterdepolarizations and triggered action potentials. In single cardiomyocytes, cardiomyocyte cell pairs, and arterially perfused tissue wedges from failing human hearts, increased hemichannel activity contributed to electrical instability compared with nonfailing rejected donor hearts. We conclude that microdomain coupling between Cx43 hemichannels and Ca2+ release is a potentially novel, targetable mechanism of cardiac arrhythmogenesis in heart failure.
Keywords: Arrhythmias; Calcium signaling; Cardiology; Cell Biology; Peptides.
Conflict of interest statement
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

