Antibody affinity versus dengue morphology influences neutralization
- PMID: 33621239
- PMCID: PMC7935256
- DOI: 10.1371/journal.ppat.1009331
Antibody affinity versus dengue morphology influences neutralization
Abstract
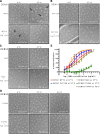
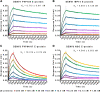
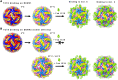
Different strains within a dengue serotype (DENV1-4) can have smooth, or "bumpy" surface morphologies with different antigenic characteristics at average body temperature (37°C). We determined the neutralizing properties of a serotype cross-reactive human monoclonal antibody (HMAb) 1C19 for strains with differing morphologies within the DENV1 and DENV2 serotypes. We mapped the 1C19 epitope to E protein domain II by hydrogen deuterium exchange mass spectrometry, cryoEM and molecular dynamics simulations, revealing that this epitope is likely partially hidden on the virus surface. We showed the antibody has high affinity for binding to recombinant DENV1 E proteins compared to those of DENV2, consistent with its strong neutralizing activities for all DENV1 strains tested regardless of their morphologies. This finding suggests that the antibody could out-compete E-to-E interaction for binding to its epitope. In contrast, for DENV2, HMAb 1C19 can only neutralize when the epitope becomes exposed on the bumpy-surfaced particle. Although HMAb 1C19 is not a suitable therapeutic candidate, this study with HMAb 1C19 shows the importance of choosing a high-affinity antibody that could neutralize diverse dengue virus morphologies for therapeutic purposes.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: J.E.C. has served as a consultant for Lilly and Luna Biologics, is a member of the Scientific Advisory Boards of CompuVax and Meissa Vaccines and is Founder of IDBiologics. The Crowe laboratory at Vanderbilt University Medical Center has received sponsored research agreements from IDBiologics and AstraZeneca.
Figures




References
-
- Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, Chua MN, et al.. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014;384(9951):1358–65. 10.1016/S0140-6736(14)61060-6 . - DOI - PubMed
-
- Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, et al.. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. The Lancet. 2012;380(9853):1559–67. 10.1016/S0140-6736(12)61428-7 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

