Immune Checkpoint Inhibitor-Related Pneumonitis in Lung Cancer: Real-World Incidence, Risk Factors, and Management Practices Across Six Health Care Centers in North Carolina
- PMID: 33621599
- PMCID: PMC8411447
- DOI: 10.1016/j.chest.2021.02.032
Immune Checkpoint Inhibitor-Related Pneumonitis in Lung Cancer: Real-World Incidence, Risk Factors, and Management Practices Across Six Health Care Centers in North Carolina
Abstract
Background: Immune checkpoint inhibitors (ICIs) are standard treatments for advanced non-small cell lung cancer and have expanded use in small cell lung cancer. Although generally better tolerated than traditional chemotherapy, immune-related adverse events, such as immune checkpoint inhibitor-related pneumonitis (ICI-P), remain poorly understood toxicities that limit ICI treatment and can result in considerable morbidity. In this retrospective case-control study, we assessed a lung cancer cohort to identify ICI-P risk factors.
Research question: What are the risk factors, clinical presentations, radiographic findings, and outcomes for ICI-P in a real-world lung cancer cohort? Do chronic pulmonary diseases confer increased risk for ICI-P?
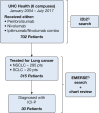
Study design and methods: Medical records from lung cancer patients receiving nivolumab, pembrolizumab, or combination ipilimumab and nivolumab at six centers in North Carolina were reviewed (January 2004-July 2017). Patients with ICI-P and control participants were characterized, and logistic regression was used to assess for ICI-P risk factors.
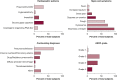
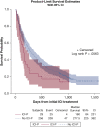
Results: Three hundred fifteen lung cancer patients who predominantly received nivolumab (76.5%) or pembrolizumab (22%) were included. The incidence of ICI-P was 9.5%, with a median time to diagnosis of 52.5 days. Most patients with ICI-P had cases of high severity, and eight patients (27%) died with ongoing ICI-P treatment. Development of ICI-P was independently associated with the presence of baseline fibrosis on chest CT scan (adjusted OR [aOR], 6.61; 95% CI, 2.48-17.7), a composite measure of obstructive lung disease (aOR, 2.79; 95% CI, 1.07-7.29), and treatment with pembrolizumab (aOR, 2.57; 95% CI, 1.08-6.11).
Interpretation: In this cohort, ICI-P was more common and severe than previously reported and carried an unexpectedly high mortality rate. Risk for ICI-P was shown to be independently associated with several chronic pulmonary diseases, which may account for the higher incidence of ICI-P in patients with lung cancer.
Keywords: immune checkpoint inhibitor; lung cancer; pneumonitis.
Copyright © 2021 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Figures
Comment in
-
Incidence of Immune Checkpoint Inhibitor-Related Pneumonitis in Lung Cancer.Chest. 2022 Mar;161(3):e196-e197. doi: 10.1016/j.chest.2021.10.041. Chest. 2022. PMID: 35256099 No abstract available.
-
Response.Chest. 2022 Mar;161(3):e197. doi: 10.1016/j.chest.2021.10.040. Chest. 2022. PMID: 35256100 No abstract available.
References
-
- Antonia S.J., Villegas A., Daniel D. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. - PubMed
-
- Hanna N.H., Schneider B.J., Temin S. Therapy for stage IV non-small-cell lung cancer without driver alterations: ASCO and OH (CCO) Joint Guideline Update. J Clin Oncol. 2020;38(14):1608–1632. - PubMed
-
- Calles A., Aguado G., Sandoval C., Álvarez R. The role of immunotherapy in small cell lung cancer. Clin Transl Oncol. 2019;21(8):961–976. - PubMed
-
- Hellmann M.D., Paz-Ares L., Bernabe Caro R. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

