Testing the effects of the GLP-1 receptor agonist exenatide on cocaine self-administration and subjective responses in humans with cocaine use disorder
- PMID: 33621809
- PMCID: PMC8026565
- DOI: 10.1016/j.drugalcdep.2021.108614
Testing the effects of the GLP-1 receptor agonist exenatide on cocaine self-administration and subjective responses in humans with cocaine use disorder
Abstract
Background: Preclinical rodent studies have demonstrated reduced cocaine taking after administration of glucagon-like peptide 1 (GLP-1) analogues. We investigated effects of a GLP-1 analogue (exenatide) on behavioral and subjective effects of cocaine in individuals with cocaine use disorder (CUD).
Methods: Non-treatment-seeking CUD subjects underwent two human laboratory cocaine self-administration test sessions following an acute 3 -h pre-treatment with exenatide (5 mcg; subcutaneously) or placebo. Primary outcomes consisted of infusions of cocaine and visual analog scale self-ratings of euphoria and wanting cocaine. Secondary outcomes consisted of pertinent hormone levels (GLP-1, insulin, and amylin).
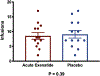
Results: Thirteen individuals completed the study. Acute pretreatment with exenatide versus placebo did not change cocaine infusions (8.5 ± 1.2 vs. 9.1 ± 1.2; p = 0.39), self-reported euphoria (4.4 ± 0.8 vs. 4.1 ± 0.8; p = 0.21), or wanting of cocaine (5.6 ± 0.9 vs. 5.4 ± 0.9; p = 0.46). Exenatide vs. placebo reduced levels of GLP-1 (p = 0.03) and insulin (p = 0.02). Self-administered cocaine also reduced levels of GLP-1 (p < 0.0001), insulin (p < 0.0001), and amylin (p < 0.0001).
Conclusions: We did not find evidence that low dose exenatide alters cocaine self-administration or the subjective effects of cocaine in people with CUD. Limitations such as single acute rather than chronic pre-treatment, as well as evaluation of only one dose, preclude drawing firm conclusions about the efficacy of exenatide. Exenatide and cocaine independently reduced levels of GLP-1 and insulin, while cocaine also reduced levels of amylin.
Keywords: Addictive behaviors; Cocaine self-administration; Cocaine use disorder; Exenatide; GLP-1; Substance-related disorders.
Copyright © 2021 Elsevier B.V. All rights reserved.
Figures


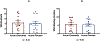
References
-
- Amato L, Minozzi S, Pani PP, Solimini R, Vecchi S, Zuccaro P, Davoli M, 2011. Dopamine agonists for the treatment of cocaine dependence. Cochrane Database Syst Rev(12), CD003352. - PubMed
-
- Amori RE, Lau J, Pittas AG, 2007. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. Jama 298(2), 194–206. - PubMed
-
- Antonsen KK, Klausen MK, Brunchmann AS, le Dous N, Jensen ME, Miskowiak KW, Fisher PM, Thomsen GK, Rindom H, Fahmy TP, Vollstaedt-Klein S, Benveniste H, Volkow ND, Becker U, Ekstrom C, Knudsen GM, Vilsboll T, Fink-Jensen A, 2018. Does glucagon-like peptide-1 (GLP-1) receptor agonist stimulation reduce alcohol intake in patients with alcohol dependence: study protocol of a randomised, double-blinded, placebo-controlled clinical trial. BMJ Open 8(7), e019562. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

