Quantitative Measurement of IgG to Severe Acute Respiratory Syndrome Coronavirus-2 Proteins Using ImmunoCAP
- PMID: 33621972
- PMCID: PMC8018212
- DOI: 10.1159/000514203
Quantitative Measurement of IgG to Severe Acute Respiratory Syndrome Coronavirus-2 Proteins Using ImmunoCAP
Abstract
Background: Detailed understanding of the immune response to severe acute respiratory syndrome coronavirus (SARS-CoV)-2, the cause of coronavirus disease 2019 (CO-VID-19) has been hampered by a lack of quantitative antibody assays.
Objective: The objective was to develop a quantitative assay for IgG to SARS-CoV-2 proteins that could be implemented in clinical and research laboratories.
Methods: The biotin-streptavidin technique was used to conjugate SARS-CoV-2 spike receptor-binding domain (RBD) or nucleocapsid protein to the solid phase of the ImmunoCAP. Plasma and serum samples from patients hospitalized with COVID-19 (n = 60) and samples from donors banked before the emergence of COVID-19 (n = 109) were used in the assay. SARS-CoV-2 IgG levels were followed longitudinally in a subset of samples and were related to total IgG and IgG to reference antigens using an ImmunoCAP 250 platform.
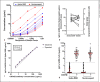
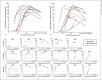
Results: At a cutoff of 2.5 μg/mL, the assay demonstrated sensitivity and specificity exceeding 95% for IgG to both SARS-CoV-2 proteins. Among 36 patients evaluated in a post-hospital follow-up clinic, median levels of IgG to spike-RBD and nucleocapsid were 34.7 μg/mL (IQR 18-52) and 24.5 μg/mL (IQR 9-59), respectively. Among 17 patients with longitudinal samples, there was a wide variation in the magnitude of IgG responses, but generally the response to spike-RBD and to nucleocapsid occurred in parallel, with peak levels approaching 100 μg/mL, or 1% of total IgG.
Conclusions: We have described a quantitative assay to measure IgG to SARS-CoV-2 that could be used in clinical and research laboratories and implemented at scale. The assay can easily be adapted to measure IgG to mutated COVID-19 proteins, has good performance characteristics, and has a readout in standardized units.
Keywords: Antibody assay; COVID-19; IgG; Nucleocapsid; Spike receptor-binding domain.
© 2021 The Author(s) Published by S. Karger AG, Basel.
Conflict of interest statement
T.P.M. has a patent on an IgE assay to α-Gal and has received assay support from Thermo Fisher Scientific. J.W. has received consultant fees and assay support from Thermo Fisher Scientific. The other authors have no conflicts of interest to declare.
Figures

Update of
-
Quantitative measurement of IgG to SARS-CoV-2 proteins using ImmunoCAP.medRxiv [Preprint]. 2020 Nov 12:2020.11.09.20228411. doi: 10.1101/2020.11.09.20228411. medRxiv. 2020. Update in: Int Arch Allergy Immunol. 2021;182(5):417-424. doi: 10.1159/000514203. PMID: 33200147 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

