The origin of animals: an ancestral reconstruction of the unicellular-to-multicellular transition
- PMID: 33622103
- PMCID: PMC8061703
- DOI: 10.1098/rsob.200359
The origin of animals: an ancestral reconstruction of the unicellular-to-multicellular transition
Abstract
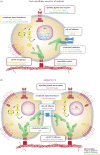
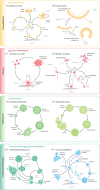
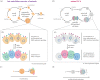
How animals evolved from a single-celled ancestor, transitioning from a unicellular lifestyle to a coordinated multicellular entity, remains a fascinating question. Key events in this transition involved the emergence of processes related to cell adhesion, cell-cell communication and gene regulation. To understand how these capacities evolved, we need to reconstruct the features of both the last common multicellular ancestor of animals and the last unicellular ancestor of animals. In this review, we summarize recent advances in the characterization of these ancestors, inferred by comparative genomic analyses between the earliest branching animals and those radiating later, and between animals and their closest unicellular relatives. We also provide an updated hypothesis regarding the transition to animal multicellularity, which was likely gradual and involved the use of gene regulatory mechanisms in the emergence of early developmental and morphogenetic plans. Finally, we discuss some new avenues of research that will complement these studies in the coming years.
Keywords: Holozoa; animal origins; cell-type evolution; evolutionary transitions; multicellularity.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
