An early Sox2-dependent gene expression programme required for hippocampal dentate gyrus development
- PMID: 33622105
- PMCID: PMC8061699
- DOI: 10.1098/rsob.200339
An early Sox2-dependent gene expression programme required for hippocampal dentate gyrus development
Abstract
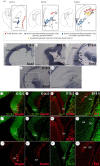
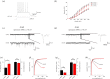
The hippocampus is a brain area central for cognition. Mutations in the human SOX2 transcription factor cause neurodevelopmental defects, leading to intellectual disability and seizures, together with hippocampal dysplasia. We generated an allelic series of Sox2 conditional mutations in mouse, deleting Sox2 at different developmental stages. Late Sox2 deletion (from E11.5, via Nestin-Cre) affects only postnatal hippocampal development; earlier deletion (from E10.5, Emx1-Cre) significantly reduces the dentate gyrus (DG), and the earliest deletion (from E9.5, FoxG1-Cre) causes drastic abnormalities, with almost complete absence of the DG. We identify a set of functionally interconnected genes (Gli3, Wnt3a, Cxcr4, p73 and Tbr2), known to play essential roles in hippocampal embryogenesis, which are downregulated in early Sox2 mutants, and (Gli3 and Cxcr4) directly controlled by SOX2; their downregulation provides plausible molecular mechanisms contributing to the defect. Electrophysiological studies of the Emx1-Cre mouse model reveal altered excitatory transmission in CA1 and CA3 regions.
Keywords: Sox; Sox2; gene regulation; mouse genetic models; transcription factors.
Figures





Similar articles
-
Deconstructing Sox2 Function in Brain Development and Disease.Cells. 2022 May 10;11(10):1604. doi: 10.3390/cells11101604. Cells. 2022. PMID: 35626641 Free PMC article. Review.
-
Tbr2 expression in Cajal-Retzius cells and intermediate neuronal progenitors is required for morphogenesis of the dentate gyrus.J Neurosci. 2013 Feb 27;33(9):4165-80. doi: 10.1523/JNEUROSCI.4185-12.2013. J Neurosci. 2013. PMID: 23447624 Free PMC article.
-
CXCR4 prevents dispersion of granule neuron precursors in the adult dentate gyrus.Hippocampus. 2013 Dec;23(12):1345-58. doi: 10.1002/hipo.22180. Epub 2013 Sep 10. Hippocampus. 2013. PMID: 23929505
-
Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh.Nat Neurosci. 2009 Oct;12(10):1248-56. doi: 10.1038/nn.2397. Epub 2009 Sep 6. Nat Neurosci. 2009. PMID: 19734891
-
COUP-TFI mitotically regulates production and migration of dentate granule cells and modulates hippocampal Cxcr4 expression.Development. 2017 Jun 1;144(11):2045-2058. doi: 10.1242/dev.139949. Epub 2017 May 15. Development. 2017. PMID: 28506990
Cited by
-
Zebrafish optic nerve regeneration involves resident and retinal oligodendrocytes.Neural Regen Res. 2026 Feb 1;21(2):811-820. doi: 10.4103/NRR.NRR-D-24-00621. Epub 2024 Oct 22. Neural Regen Res. 2026. PMID: 39878527 Free PMC article.
-
SOX2-Sensing: Insights into the Role of SOX2 in the Generation of Sensory Cell Types in Vertebrates.Int J Mol Sci. 2023 Apr 21;24(8):7637. doi: 10.3390/ijms24087637. Int J Mol Sci. 2023. PMID: 37108798 Free PMC article. Review.
-
Deconstructing Sox2 Function in Brain Development and Disease.Cells. 2022 May 10;11(10):1604. doi: 10.3390/cells11101604. Cells. 2022. PMID: 35626641 Free PMC article. Review.
-
FOS Rescues Neuronal Differentiation of Sox2-Deleted Neural Stem Cells by Genome-Wide Regulation of Common SOX2 and AP1(FOS-JUN) Target Genes.Cells. 2021 Jul 12;10(7):1757. doi: 10.3390/cells10071757. Cells. 2021. PMID: 34359927 Free PMC article.
-
Bridging between Mouse and Human Enhancer-Promoter Long-Range Interactions in Neural Stem Cells, to Understand Enhancer Function in Neurodevelopmental Disease.Int J Mol Sci. 2022 Jul 19;23(14):7964. doi: 10.3390/ijms23147964. Int J Mol Sci. 2022. PMID: 35887306 Free PMC article.
References
-
- Kandel ER, Schwartz JH, Jessell TM. 2000. Principles of neural science, 4th edn. New York, NY: McGraw-Hill, Health Professions Division.
-
- Kondoh H L-BReb. 2016. Sox2, biology and role in development and disease. London, UK: Academic Press.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

