Transcriptomics analysis of Toxoplasma gondii-infected mouse macrophages reveals coding and noncoding signatures in the presence and absence of MyD88
- PMID: 33622246
- PMCID: PMC7903719
- DOI: 10.1186/s12864-021-07437-0
Transcriptomics analysis of Toxoplasma gondii-infected mouse macrophages reveals coding and noncoding signatures in the presence and absence of MyD88
Abstract
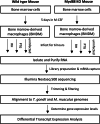
Background: Toxoplasma gondii is a globally distributed protozoan parasite that establishes life-long asymptomatic infection in humans, often emerging as a life-threatening opportunistic pathogen during immunodeficiency. As an intracellular microbe, Toxoplasma establishes an intimate relationship with its host cell from the outset of infection. Macrophages are targets of infection and they are important in early innate immunity and possibly parasite dissemination throughout the host. Here, we employ an RNA-sequencing approach to identify host and parasite transcriptional responses during infection of mouse bone marrow-derived macrophages (BMDM). We incorporated into our analysis infection with the high virulence Type I RH strain and the low virulence Type II strain PTG. Because the well-known TLR-MyD88 signaling axis is likely of less importance in humans, we examined transcriptional responses in both MyD88+/+ and MyD88-/- BMDM. Long noncoding (lnc) RNA molecules are emerging as key regulators in infection and immunity, and were, therefore, included in our analysis.
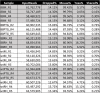
Results: We found significantly more host genes were differentially expressed in response to the highly virulent RH strain rather than with the less virulent PTG strain (335 versus 74 protein coding genes for RH and PTG, respectively). Enriched in these protein coding genes were subsets associated with the immune response as well as cell adhesion and migration. We identified 249 and 83 non-coding RNAs as differentially expressed during infection with RH and PTG strains, respectively. Although the majority of these are of unknown function, one conserved lncRNA termed mir17hg encodes the mir17 microRNA gene cluster that has been implicated in down-regulating host cell apoptosis during T. gondii infection. Only a minimal number of transcripts were differentially expressed between MyD88 knockout and wild type cells. However, several immune genes were among the differences. While transcripts for parasite secretory proteins were amongst the most highly expressed T. gondii genes during infection, no differentially expressed parasite genes were identified when comparing infection in MyD88 knockout and wild type host BMDM.
Conclusions: The large dataset presented here lays the groundwork for continued studies on both the MyD88-independent immune response and the function of lncRNAs during Toxoplasma gondii infection.
Keywords: Macrophages; MyD88; Noncoding RNA; Parasite; Toxoplasma gondii; lncRNA.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







Similar articles
-
Toxoplasma gondii Manipulates Expression of Host Long Noncoding RNA during Intracellular Infection.Sci Rep. 2018 Oct 9;8(1):15017. doi: 10.1038/s41598-018-33274-5. Sci Rep. 2018. PMID: 30301916 Free PMC article.
-
T lymphocyte-dependent IL-10 down-regulates a cytokine storm driven by Toxoplasma gondii GRA24.mBio. 2024 Nov 13;15(11):e0145524. doi: 10.1128/mbio.01455-24. Epub 2024 Oct 23. mBio. 2024. PMID: 39440975 Free PMC article.
-
Toxoplasma gondii dense granule protein GRA24 drives MyD88-independent p38 MAPK activation, IL-12 production and induction of protective immunity.PLoS Pathog. 2020 May 15;16(5):e1008572. doi: 10.1371/journal.ppat.1008572. eCollection 2020 May. PLoS Pathog. 2020. PMID: 32413093 Free PMC article.
-
Transcriptomic profiling of long non-coding RNAs and messenger RNAs in the liver of mice during Toxoplasma gondii infection.Parasit Vectors. 2024 Jan 16;17(1):20. doi: 10.1186/s13071-023-06053-z. Parasit Vectors. 2024. PMID: 38229193 Free PMC article. Review.
-
Impact of Toxoplasma gondii Infection on Host Non-coding RNA Responses.Front Cell Infect Microbiol. 2019 May 14;9:132. doi: 10.3389/fcimb.2019.00132. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31157172 Free PMC article. Review.
Cited by
-
CircRNA and miRNA expression analysis in livers of mice with Toxoplasma gondii infection.Front Cell Infect Microbiol. 2022 Oct 27;12:1037586. doi: 10.3389/fcimb.2022.1037586. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36389171 Free PMC article.
-
A Systematic Review of Apicomplexa Looking into Epigenetic Pathways and the Opportunity for Novel Therapies.Pathogens. 2023 Feb 11;12(2):299. doi: 10.3390/pathogens12020299. Pathogens. 2023. PMID: 36839571 Free PMC article. Review.
-
Transcriptomics of Besnoitia besnoiti-Infected Fibroblasts Reveals Hallmarks of Early Fibrosis and Cancer Progression.Microorganisms. 2024 Mar 15;12(3):586. doi: 10.3390/microorganisms12030586. Microorganisms. 2024. PMID: 38543637 Free PMC article.
-
scDual-Seq of Toxoplasma gondii-infected mouse BMDCs reveals heterogeneity and differential infection dynamics.Front Immunol. 2023 Jul 27;14:1224591. doi: 10.3389/fimmu.2023.1224591. eCollection 2023. Front Immunol. 2023. PMID: 37575232 Free PMC article.
-
Functional Intricacy and Symmetry of Long Non-Coding RNAs in Parasitic Infections.Front Cell Infect Microbiol. 2021 Oct 8;11:751523. doi: 10.3389/fcimb.2021.751523. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34692567 Free PMC article. Review.
References
-
- Dubey JP. The history and life-cycle of Toxoplasma gondii. In: Weiss LM, Kim K, editors. Toxoplasma gondii the model apicomplexan: perspective and methods. 2. San Diego: Academic Press; 2013. pp. 1–17.
-
- McLeod RM, van Tubbergen C, Montoya JG, Petersen E. Human toxoplasma infection. In: Weiss LM, Kim K, editors. Toxoplasma gondii the model Apicomplexan: perspectives and methods. 2. San Diego: Academic Press; 2013. pp. 100–159.
-
- Pfaff AW, Liesenfeld O, Candolfi E. Congenital toxoplasmosis. In: Ajioka JW, Soldati D, editors. Toxoplasma molecular and cellular biology. Norfolk: Horizon Bioscience; 2007. pp. 93–110.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

