Randomized crossover trial of a modified ketogenic diet in Alzheimer's disease
- PMID: 33622392
- PMCID: PMC7901512
- DOI: 10.1186/s13195-021-00783-x
Randomized crossover trial of a modified ketogenic diet in Alzheimer's disease
Abstract
Background: Brain energy metabolism is impaired in Alzheimer's disease (AD), which may be mitigated by a ketogenic diet. We conducted a randomized crossover trial to determine whether a 12-week modified ketogenic diet improved cognition, daily function, or quality of life in a hospital clinic of AD patients.
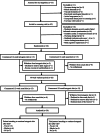
Methods: We randomly assigned patients with clinically confirmed diagnoses of AD to a modified ketogenic diet or usual diet supplemented with low-fat healthy-eating guidelines and enrolled them in a single-phase, assessor-blinded, two-period crossover trial (two 12-week treatment periods, separated by a 10-week washout period). Primary outcomes were mean within-individual changes in the Addenbrookes Cognitive Examination - III (ACE-III) scale, AD Cooperative Study - Activities of Daily Living (ADCS-ADL) inventory, and Quality of Life in AD (QOL-AD) questionnaire over 12 weeks. Secondary outcomes considered changes in cardiovascular risk factors and adverse effects.
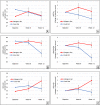
Results: We randomized 26 patients, of whom 21 (81%) completed the ketogenic diet; only one withdrawal was attributed to the ketogenic diet. While on the ketogenic diet, patients achieved sustained physiological ketosis (12-week mean beta-hydroxybutyrate level: 0.95 ± 0.34 mmol/L). Compared with usual diet, patients on the ketogenic diet increased their mean within-individual ADCS-ADL (+ 3.13 ± 5.01 points, P = 0.0067) and QOL-AD (+ 3.37 ± 6.86 points, P = 0.023) scores; the ACE-III also increased, but not significantly (+ 2.12 ± 8.70 points, P = 0.24). Changes in cardiovascular risk factors were mostly favourable, and adverse effects were mild.
Conclusions: This is the first randomized trial to investigate the impact of a ketogenic diet in patients with uniform diagnoses of AD. High rates of retention, adherence, and safety appear to be achievable in applying a 12-week modified ketogenic diet to AD patients. Compared with a usual diet supplemented with low-fat healthy-eating guidelines, patients on the ketogenic diet improved in daily function and quality of life, two factors of great importance to people living with dementia.
Trial registration: This trial is registered on the Australia New Zealand Clinical Trials Registry, number ACTRN12618001450202 . The trial was registered on August 28, 2018.
Keywords: Alzheimer’s disease; Cognition; Daily function; Ketogenic diet; Quality of life; Randomized crossover trial.
Conflict of interest statement
The authors of this trial report no conflicts of interest. DKJM runs a whole-foods coaching business; however, none of her recipes were used in this trial. All recipes were obtained from sources with no personal or financial affiliation to any of the authors.
Figures

References
-
- Alzheimer’s Disease International. The World Alzheimer Report 2019: Attitudes to dementia. London, UK, 2019. https://www.alz.co.uk/research/world-report-2019. Accessed 24 May 2020
-
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

