Frontotemporal lobar degeneration proteinopathies have disparate microscopic patterns of white and grey matter pathology
- PMID: 33622418
- PMCID: PMC7901087
- DOI: 10.1186/s40478-021-01129-2
Frontotemporal lobar degeneration proteinopathies have disparate microscopic patterns of white and grey matter pathology
Abstract
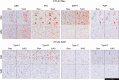
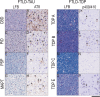
Frontotemporal lobar degeneration proteinopathies with tau inclusions (FTLD-Tau) or TDP-43 inclusions (FTLD-TDP) are associated with clinically similar phenotypes. However, these disparate proteinopathies likely differ in cellular severity and regional distribution of inclusions in white matter (WM) and adjacent grey matter (GM), which have been understudied. We performed a neuropathological study of subcortical WM and adjacent GM in a large autopsy cohort (n = 92; FTLD-Tau = 37, FTLD-TDP = 55) using a validated digital image approach. The antemortem clinical phenotype was behavioral-variant frontotemporal dementia (bvFTD) in 23 patients with FTLD-Tau and 42 with FTLD-TDP, and primary progressive aphasia (PPA) in 14 patients with FTLD-Tau and 13 with FTLD-TDP. We used linear mixed-effects models to: (1) compare WM pathology burden between proteinopathies; (2) investigate the relationship between WM pathology burden and WM degeneration using luxol fast blue (LFB) myelin staining; (3) study regional patterns of pathology burden in clinico-pathological groups. WM pathology burden was greater in FTLD-Tau compared to FTLD-TDP across regions (beta = 4.21, SE = 0.34, p < 0.001), and correlated with the degree of WM degeneration in both FTLD-Tau (beta = 0.32, SE = 0.10, p = 0.002) and FTLD-TDP (beta = 0.40, SE = 0.08, p < 0.001). WM degeneration was greater in FTLD-Tau than FTLD-TDP particularly in middle-frontal and anterior cingulate regions (p < 0.05). Distinct regional patterns of WM and GM inclusions characterized FTLD-Tau and FTLD-TDP proteinopathies, and associated in part with clinical phenotype. In FTLD-Tau, WM pathology was particularly severe in the dorsolateral frontal cortex in nonfluent-variant PPA, and GM pathology in dorsolateral and paralimbic frontal regions with some variation across tauopathies. Differently, FTLD-TDP had little WM regional variability, but showed severe GM pathology burden in ventromedial prefrontal regions in both bvFTD and PPA. To conclude, FTLD-Tau and FTLD-TDP proteinopathies have distinct severity and regional distribution of WM and GM pathology, which may impact their clinical presentation, with overall greater severity of WM pathology as a distinguishing feature of tauopathies.
Keywords: Frontotemporal dementia; Neuropathology; Primary progressive aphasia; TDP-43; Tau.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures



References
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. - DOI - PubMed
-
- Boluda S, Iba M, Zhang B, Raible KM, Lee VM, Trojanowski JQ. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer's disease or corticobasal degeneration brains. Acta Neuropathol. 2015;129:221–237. doi: 10.1007/s00401-014-1373-0. - DOI - PMC - PubMed
-
- Boxer AL, Gold M, Huey E, Hu WT, Rosen H, Kramer J, Gao FB, Burton EA, Chow T, Kao A, et al. The advantages of frontotemporal degeneration drug development (part 2 of frontotemporal degeneration: the next therapeutic frontier) Alzheimer's Dementia J Alzheimer's Assoc. 2012 doi: 10.1016/j.jalz.2012.03.003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

