Glucocorticoids alone versus tocilizumab alone or glucocorticoids plus tocilizumab in patients with severe SARS-CoV-2 pneumonia and mild inflammation
- PMID: 33622529
- PMCID: PMC7843156
- DOI: 10.1016/j.medcli.2021.01.006
Glucocorticoids alone versus tocilizumab alone or glucocorticoids plus tocilizumab in patients with severe SARS-CoV-2 pneumonia and mild inflammation
Abstract
Aim: To assess clinical outcomes according to the immunosuppressive treatment administered to patients with severe SARS-CoV-2 pneumonia and moderate inflammation.
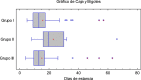
Methods: A retrospective observational cohort study involving 142 patients with severe COVID-19 pneumonia and moderate inflammation divided into three treatment groups (pulses of methylprednisolone alone [groupI], tocilizumab alone [groupII] and methylprednisolone plus tocilizumab [groupIII]). The aim was to assess intergroups differences in the clinical course with a 60-day follow-up and related analytical factors.
Results: 14 patients (9,8%) died: 8 (10%) in groupI and 6 (9,5%) in groupsII andIII. 15 (10,6%) were admitted to ICU: 2 (2,5%) from groupI, 4 (28,5%) from groupII and 9 (18,4%) from groupIII. The mean hospital stay was longer in groupII and clinical outcome was not associated with treatment.
Conclusions: Tocilizumab seems to be not associated with better clinical outcomes and should be reserved for clinical trial scenario, since its widespread use may result in higher rate of ICU admission and longer mean hospital stay without differences in mortality rate and potentially adverse events.
Keywords: COVID-19; Cytokine storm syndrome; Inflamación; Inflammation; Methylprednisolone; Metilprednisolona; Síndrome de tormenta de citoquinas; Tocilizumab.
Copyright © 2021 Elsevier España, S.L.U. All rights reserved.
Figures
Similar articles
-
Glucocorticoids alone versus tocilizumab alone or glucocorticoids plus tocilizumab in patients with severe SARS-CoV-2 pneumonia and mild inflammation.Med Clin (Engl Ed). 2021 Jun 25;156(12):602-605. doi: 10.1016/j.medcle.2021.01.004. Epub 2021 May 24. Med Clin (Engl Ed). 2021. PMID: 34056111 Free PMC article.
-
Intravenous methylprednisolone with or without tocilizumab in patients with severe COVID-19 pneumonia requiring oxygen support: A prospective comparison.J Infect Public Health. 2021 Aug;14(8):985-989. doi: 10.1016/j.jiph.2021.06.003. Epub 2021 Jun 10. J Infect Public Health. 2021. PMID: 34153729 Free PMC article. Clinical Trial.
-
Tocilizumab or glucocorticoids treatment for patients with SARS-CoV-2 pneumonia: An observational study.Braz J Infect Dis. 2022 Jan-Feb;26(1):101702. doi: 10.1016/j.bjid.2021.101702. Epub 2021 Dec 21. Braz J Infect Dis. 2022. PMID: 34963560 Free PMC article.
-
Tocilizumab in SARS-CoV-2 Patients with the Syndrome of Cytokine Storm: A Narrative Review.Rev Recent Clin Trials. 2021;16(2):138-145. doi: 10.2174/1574887115666200917110954. Rev Recent Clin Trials. 2021. PMID: 32940187 Review.
-
Immunomodulation for the management of severe SARS-CoV2 infections. State of the art and review of the literature.Biochem Biophys Res Commun. 2021 Jan 29;538:151-155. doi: 10.1016/j.bbrc.2020.11.084. Epub 2020 Nov 28. Biochem Biophys Res Commun. 2021. PMID: 33303188 Free PMC article. Review.
References
-
- Ramiro S., Mostard R.L.M., Magro-Checa C., van Dongen C.M.P., Dormans T., Buijs J., et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: Results of the CHIC study. Ann Rheum Dis. 2020;79:1143–1151. doi: 10.1136/annrheumdis-2020-218479. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous