Inhaled Cannabis Suppresses Chemotherapy-Induced Neuropathic Nociception by Decoupling the Raphe Nucleus: A Functional Imaging Study in Rats
- PMID: 33622657
- PMCID: PMC8351528
- DOI: 10.1016/j.bpsc.2020.11.015
Inhaled Cannabis Suppresses Chemotherapy-Induced Neuropathic Nociception by Decoupling the Raphe Nucleus: A Functional Imaging Study in Rats
Abstract
Background: Efficacy of inhaled cannabis for treating pain is controversial. Effective treatment for chemotherapy-induced neuropathy represents an unmet medical need. We hypothesized that cannabis reduces neuropathic pain by reducing functional coupling in the raphe nuclei.
Methods: We assessed the impact of inhalation of vaporized cannabis plant (containing 10.3% Δ9-tetrahydrocannabinol/0.05% cannabidiol) or placebo cannabis on brain resting-state blood oxygen level-dependent functional connectivity and pain behavior induced by paclitaxel in rats. Rats received paclitaxel to produce chemotherapy-induced peripheral neuropathy or its vehicle. Behavioral and imaging experiments were performed after neuropathy was established and stable. Images were registered to, and analyzed using, a 3D magnetic resonance imaging rat atlas providing site-specific data on more than 168 different brain areas.
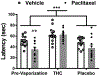
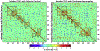
Results: Prior to vaporization, paclitaxel produced cold allodynia. Inhaled vaporized cannabis increased cold withdrawal latencies relative to prevaporization or placebo cannabis, consistent with Δ9-tetrahydrocannabinol-induced antinociception. In paclitaxel-treated rats, the midbrain serotonergic system, comprising the dorsal and median raphe, showed hyperconnectivity to cortical, brainstem, and hippocampal areas, consistent with nociceptive processing. Inhalation of vaporized cannabis uncoupled paclitaxel-induced hyperconnectivity patterns. No such changes in connectivity or cold responsiveness were observed following placebo cannabis vaporization.
Conclusions: Inhaled vaporized cannabis plant uncoupled brain resting-state connectivity in the raphe nuclei, normalizing paclitaxel-induced hyperconnectivity to levels observed in vehicle-treated rats. Inhaled vaporized cannabis produced antinociception in both paclitaxel- and vehicle-treated rats. Our study elucidates neural circuitry implicated in the therapeutic effects of Δ9-tetrahydrocannabinol and supports a role for functional imaging studies in animals in guiding indications for future clinical trials.
Keywords: Cold allodynia; Hyperconnectivity; Paclitaxel; Phytocannabinoids; Resting-state BOLD functional connectivity; Serotonin; Tetrahydrocannabinol; Vaporized marijuana plant.
Copyright © 2020 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosures
CFF has a financial interest in Animal Imaging Research, the company that makes the RF electronics and holders for animal imaging. All other authors report no biomedical financial interests or potential conflicts of interest.
Figures
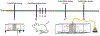


References
-
- Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, et al., Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis, Pain 155,2014, 2461–2470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

