Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study
- PMID: 33622706
- PMCID: PMC8382991
- DOI: 10.1158/1078-0432.CCR-20-4573
Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study
Abstract
Purpose: Current FDA-approved imaging modalities are inadequate for localizing prostate cancer biochemical recurrence (BCR). 18F-DCFPyL is a highly selective, small-molecule prostate-specific membrane antigen-targeted PET radiotracer. CONDOR was a prospective study designed to determine the performance of 18F-DCFPyL-PET/CT in patients with BCR and uninformative standard imaging.
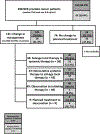
Experimental design: Men with rising PSA ≥0.2 ng/mL after prostatectomy or ≥2 ng/mL above nadir after radiotherapy were eligible. The primary endpoint was correct localization rate (CLR), defined as positive predictive value with an additional requirement of anatomic lesion colocalization between 18F-DCFPyL-PET/CT and a composite standard of truth (SOT). The SOT consisted of, in descending priority (i) histopathology, (ii) subsequent correlative imaging findings, or (iii) post-radiation PSA response. The trial was considered a success if the lower bound of the 95% confidence interval (CI) for CLR exceeded 20% for two of three 18F-DCFPyL-PET/CT readers. Secondary endpoints included change in intended management and safety.
Results: A total of 208 men with a median baseline PSA of 0.8 ng/mL (range: 0.2-98.4 ng/mL) underwent 18F-DCFPyL-PET/CT. The CLR was 84.8%-87.0% (lower bound of 95% CI: 77.8-80.4). A total of 63.9% of evaluable patients had a change in intended management after 18F-DCFPyL-PET/CT. The disease detection rate was 59% to 66% (at least one lesion detected per patient by 18F-DCFPyL-PET/CT by central readers).
Conclusions: Performance of 18F-DCFPyL-PET/CT achieved the study's primary endpoint, demonstrating disease localization in the setting of negative standard imaging and providing clinically meaningful and actionable information. These data further support the utility of 18F-DCFPyL-PET/CT to localize disease in men with recurrent prostate cancer.See related commentary by True and Chen, p. 3512.
©2021 American Association for Cancer Research.
Conflict of interest statement
Conflict of interest statement: COI’s have been submitted electronically.
Figures



Comment in
-
How Accurately does PSMA Inhibitor 18F-DCFPyL-PET-CT Image Prostate Cancer?Clin Cancer Res. 2021 Jul 1;27(13):3512-3514. doi: 10.1158/1078-0432.CCR-21-0749. Epub 2021 Apr 23. Clin Cancer Res. 2021. PMID: 33893158 Free PMC article.
-
Urological Oncology: Prostate Cancer.J Urol. 2021 Oct;206(4):1062-1064. doi: 10.1097/JU.0000000000002132. Epub 2021 Jul 20. J Urol. 2021. PMID: 34281353 No abstract available.
-
Imaging Biochemical Recurrence in Prostate Cancer.Radiol Imaging Cancer. 2021 Jul;3(4):e219015. doi: 10.1148/rycan.2021219015. Radiol Imaging Cancer. 2021. PMID: 34328353 Free PMC article. No abstract available.
References
-
- Amling CL, Blute ML, Bergstralh EJ, Seay TM, Slezak J, Zincke H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. J Urol 2000; 164: 101–105. - PubMed
-
- Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol 2003; 169: 517–523. - PubMed
-
- Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005; 294: 433–439. - PubMed
-
- Kestin LL, Vicini FA, Ziaja EL, Stromberg JS, Frazier RC, Martinez AA. Defining biochemical cure for prostate carcinoma patients treated with external beam radiation therapy. Cancer 1999; 86: 1557–1566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

