Performance and Implementation Evaluation of the Abbott BinaxNOW Rapid Antigen Test in a High-Throughput Drive-Through Community Testing Site in Massachusetts
- PMID: 33622768
- PMCID: PMC8091851
- DOI: 10.1128/JCM.00083-21
Performance and Implementation Evaluation of the Abbott BinaxNOW Rapid Antigen Test in a High-Throughput Drive-Through Community Testing Site in Massachusetts
Abstract
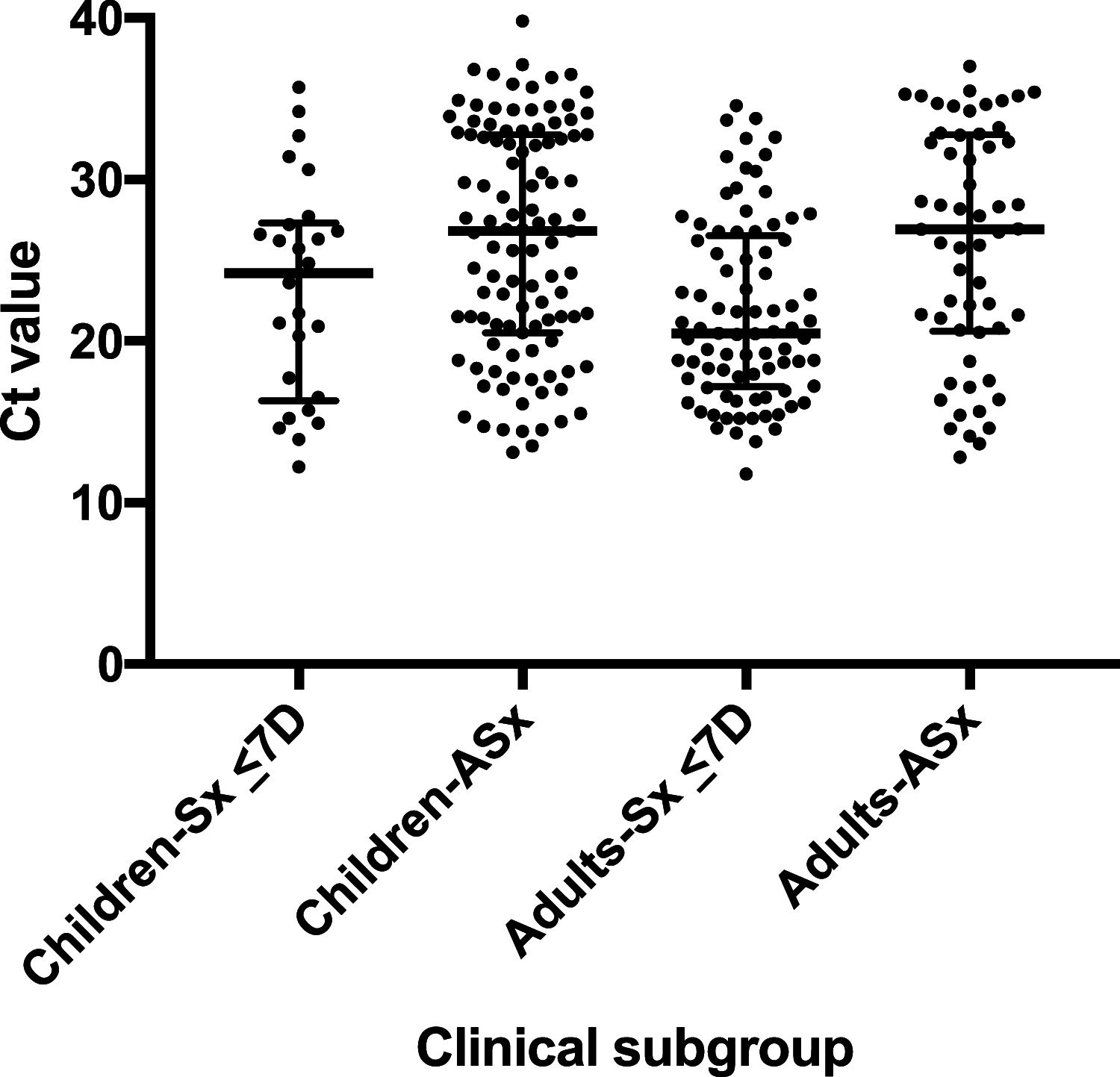
Rapid diagnostic tests (RDTs) for SARS-CoV-2 antigens (Ag) that can be performed at point of care (POC) can supplement molecular testing and help mitigate the COVID-19 pandemic. Deployment of an Ag RDT requires an understanding of its operational and performance characteristics under real-world conditions and in relevant subpopulations. We evaluated the Abbott BinaxNOW COVID-19 Ag card in a high-throughput, drive-through, free community testing site in Massachusetts using anterior nasal (AN) swab reverse transcriptase PCR (RT-PCR) for clinical testing. Individuals presenting for molecular testing in two of seven lanes were offered the opportunity to also receive BinaxNOW testing. Dual AN swabs were collected from symptomatic and asymptomatic children (≤18 years of age) and adults. BinaxNOW testing was performed in a testing pod with temperature/humidity monitoring. One individual performed testing and official result reporting for each test, but most tests had a second independent reading to assess interoperator agreement. Positive BinaxNOW results were scored as faint, medium, or strong. Positive BinaxNOW results were reported to patients by phone, and they were instructed to isolate pending RT-PCR results. The paired RT-PCR result was the reference for sensitivity and specificity calculations. Of 2,482 participants, 1,380 adults and 928 children had paired RT-PCR/BinaxNOW results and complete symptom data. In this study, 974/1,380 (71%) adults and 829/928 (89%) children were asymptomatic. BinaxNOW had 96.5% (95% confidence interval [CI], 90.0 to 99.3) sensitivity and 100% (95% CI, 98.6 to 100.0) specificity in adults within 7 days of symptoms and 84.6% (95% CI, 65.1 to 95.6) sensitivity and 100% (95% CI, 94.5 to 100.0) specificity in children within 7 days of symptoms. Sensitivity and specificity in asymptomatic adults were 70.2% (95% CI, 56.6 to 81.6) and 99.6% (95% CI, 98.9 to 99.9), respectively, and in asymptomatic children, they were 65.4% (95% CI, 55.6 to 74.4) and 99.0% (95% CI, 98.0 to 99.6), respectively. By cycle threshold (CT ) value cutoff, sensitivity in all subgroups combined (n = 292 RT-PCR-positive individuals) was 99.3% with CT values of ≤25, 95.8% with CT values of ≤30, and 81.2% with CT values of ≤35. Twelve false-positive BinaxNOW results (out of 2,308 tests) were observed; in all 12, the test bands were faint but otherwise normal and were noted by both readers. One invalid BinaxNOW result was identified. Interoperator agreement (positive versus negative BinaxNOW result) was 100% (n = 2,230/2,230 double reads). Each operator was able to process 20 RDTs per hour. In a separate set of 30 specimens (from individuals with symptoms ≤7 days) run at temperatures below the manufacturer's recommended range (46 to 58.5°F), sensitivity was 66.7% and specificity 95.2%. BinaxNOW had very high specificity in both adults and children and very high sensitivity in newly symptomatic adults. Overall, 95.8% sensitivity was observed with CT values of ≤30. These data support public health recommendations for use of the BinaxNOW test in adults with symptoms for ≤7 days without RT-PCR confirmation. Excellent interoperator agreement indicates that an individual can perform and read the BinaxNOW test alone. A skilled laboratorian can perform and read 20 tests per hour. Careful attention to temperature is critical.
Keywords: COVID-19; SARS-CoV-2; antigen; diagnostic; point of care.
Copyright © 2021 American Society for Microbiology.
Figures
References
-
- Krüger LJ, Gaeddert M, Köppel L, Brümmer LE, Gottschalk C, Miranda IB, Schnitzler P, Kräusslich HG, Lindner AK, Nikolai O, Mockenhaupt FP, Seybold J, Corman VM, Drosten C, Pollock NR, Cubas-Atienzar AI, Kontogianni K, Collins A, Wright AH, Knorr B, Welker A, de Vos M, Sacks JA, Adams ER, Denkinger CM. 2020. Evaluation of the accuracy, ease of use and limit of detection of novel, rapid, antigen-detecting point-of-care diagnostics for SARS-CoV-2. medRxiv 10.1101/2020.10.01.20203836. - DOI
-
- Lindner AK, Nikolai O, Kausch F, Wintel M, Hommes F, Gertler M, Krüger L, Gaeddert M, Tobian F, Lainati F, Köppel L, Seybold J, Corman VM, Drosten C, Hofmann J, Sacks J, Mockenhaupt F, Denkinger CM. 2020. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with self-collected anterior nasal swab versus professional-collected nasopharyngeal swab. Eur Respir J, in press. 10.1183/13993003.03961-2020. - DOI - PMC - PubMed
-
- Becton Dickinson and Company. 2021. Package insert for the BD VeritorTM system for rapid detection of SARS-CoV-2. https://www.fda.gov/media/139755/download.
-
- Quidel Corporation. 2020. Package insert for the Sofia SARS antigen FIA test. https://www.fda.gov/media/137885/download.
-
- Abbott Laboratories. 2020. Package insert for the Abbott BinaxNOW COVID-19 Ag card. https://www.fda.gov/media/141570/download.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

