Efficacy of 177Lu-Dotatate Induction and Maintenance Therapy of Various Types of Neuroendocrine Tumors: A Phase II Registry Study
- PMID: 33622997
- PMCID: PMC7816182
- DOI: 10.3390/curroncol28010015
Efficacy of 177Lu-Dotatate Induction and Maintenance Therapy of Various Types of Neuroendocrine Tumors: A Phase II Registry Study
Abstract
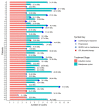
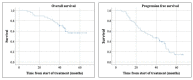
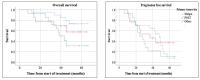
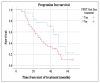
Peptide receptor radionuclide therapy (PRRT) has been recently established as a treatment option for progressive gastro-entero-pancreatic neuroendocrine tumors (NETs) including four 200 mCi induction cycles. The purpose of this phase 2 trial is to expand use of PRRT to different types of NETs with the application of dose adjustment and evaluate value of maintenance therapy in patients who had disease control on induction therapy. Forty-seven PRRT naïve NET patients with different primary origin received 177Lu-DOTATATE induction therapy, ranging from 75 to 150 mCi per cycle, based on patients' clinical status such as liver and renal function, extent of metastases, and previous therapies. Thirty-four patients underwent additional maintenance therapy (50-100 mCi per cycle) following induction course until they developed disease progression. The estimated median progression-free survival (PFS) was 36.1 months. The median PFS in our MNET subgroup was 47.7 months, which is markedly longer than NETTER-1 trial with median PFS of 28.4 months. The median PFS was significantly longer in patients who received PRRT as first-line treatment after disease progression on somatostatin analogs compared to patients who received other therapies first (p-value = 0.04). The total disease response rate (DRR) and disease control rate (DCR) was 32% and 85% based on RECIST 1.1 and 45% and 83% based on Choi criteria. This trial demonstrates longer PFS with the addition of low dose maintenance therapy to induction therapy compared to NETTER-1 trial that only included induction therapy. Also, we observed considerable efficacy of PRRT in various types of advanced NETs.
Keywords: DOTATAE; Lu-177; NET; PRRT; neuroendocrine tumor; peptide receptor radionuclide therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Cavalcoli F., Garrahy A., Castellaneta M., Tamagno G. Epidemiology of neuroendocrine tumours. Neuroendocrinology. 2004;80(Suppl. 1):3–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

