Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson's disease by regulating gut microbiota
- PMID: 33623004
- PMCID: PMC7902645
- DOI: 10.1038/s41392-020-00456-5
Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson's disease by regulating gut microbiota
Abstract
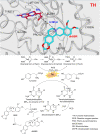
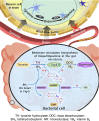
The phenylalanine-tyrosine-dopa-dopamine pathway provides dopamine to the brain. In this process, tyrosine hydroxylase (TH) is the rate-limiting enzyme that hydroxylates tyrosine and generates levodopa (L-dopa) with tetrahydrobiopterin (BH4) as a coenzyme. Here, we show that oral berberine (BBR) might supply H• through dihydroberberine (reduced BBR produced by bacterial nitroreductase) and promote the production of BH4 from dihydrobiopterin; the increased BH4 enhances TH activity, which accelerates the production of L-dopa by the gut bacteria. Oral BBR acts in a way similar to vitamins. The L-dopa produced by the intestinal bacteria enters the brain through the circulation and is transformed to dopamine. To verify the gut-brain dialog activated by BBR's effect, Enterococcus faecalis or Enterococcus faecium was transplanted into Parkinson's disease (PD) mice. The bacteria significantly increased brain dopamine and ameliorated PD manifestation in mice; additionally, combination of BBR with bacteria showed better therapeutic effect than that with bacteria alone. Moreover, 2,4,6-trimethyl-pyranylium tetrafluoroborate (TMP-TFB)-derivatized matrix-assisted laser desorption mass spectrometry (MALDI-MS) imaging of dopamine identified elevated striatal dopamine levels in mouse brains with oral Enterococcus, and BBR strengthened the imaging intensity of brain dopamine. These results demonstrated that BBR was an agonist of TH in Enterococcus and could lead to the production of L-dopa in the gut. Furthermore, a study of 28 patients with hyperlipidemia confirmed that oral BBR increased blood/fecal L-dopa by the intestinal bacteria. Hence, BBR might improve the brain function by upregulating the biosynthesis of L-dopa in the gut microbiota through a vitamin-like effect.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

