Climate predicts geographic and temporal variation in mosquito-borne disease dynamics on two continents
- PMID: 33623008
- PMCID: PMC7902664
- DOI: 10.1038/s41467-021-21496-7
Climate predicts geographic and temporal variation in mosquito-borne disease dynamics on two continents
Abstract
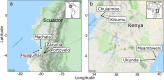
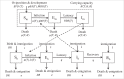
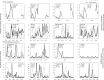
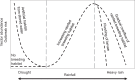
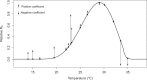
Climate drives population dynamics through multiple mechanisms, which can lead to seemingly context-dependent effects of climate on natural populations. For climate-sensitive diseases, such as dengue, chikungunya, and Zika, climate appears to have opposing effects in different contexts. Here we show that a model, parameterized with laboratory measured climate-driven mosquito physiology, captures three key epidemic characteristics across ecologically and culturally distinct settings in Ecuador and Kenya: the number, timing, and duration of outbreaks. The model generates a range of disease dynamics consistent with observed Aedes aegypti abundances and laboratory-confirmed arboviral incidence with variable accuracy (28-85% for vectors, 44-88% for incidence). The model predicted vector dynamics better in sites with a smaller proportion of young children in the population, lower mean temperature, and homes with piped water and made of cement. Models with limited calibration that robustly capture climate-virus relationships can help guide intervention efforts and climate change disease projections.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Burkett VR, et al. Nonlinear dynamics in ecosystem response to climatic change: case studies and policy implications. Ecol. Complex. 2005;2:357–394. doi: 10.1016/j.ecocom.2005.04.010. - DOI
-
- Molnár, P. K., Sckrabulis, J. P., Altman, K. A. & Raffel, T. R. Thermal performance curves and the metabolic theory of ecology—a practical guide to models and experiments for parasitologists. J. Parasitol.103, 423–439 (2017). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

