A stable cathode-solid electrolyte composite for high-voltage, long-cycle-life solid-state sodium-ion batteries
- PMID: 33623048
- PMCID: PMC7902639
- DOI: 10.1038/s41467-021-21488-7
A stable cathode-solid electrolyte composite for high-voltage, long-cycle-life solid-state sodium-ion batteries
Abstract
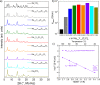
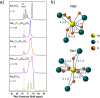
Rechargeable solid-state sodium-ion batteries (SSSBs) hold great promise for safer and more energy-dense energy storage. However, the poor electrochemical stability between current sulfide-based solid electrolytes and high-voltage oxide cathodes has limited their long-term cycling performance and practicality. Here, we report the discovery of the ion conductor Na3-xY1-xZrxCl6 (NYZC) that is both electrochemically stable (up to 3.8 V vs. Na/Na+) and chemically compatible with oxide cathodes. Its high ionic conductivity of 6.6 × 10-5 S cm-1 at ambient temperature, several orders of magnitude higher than oxide coatings, is attributed to abundant Na vacancies and cooperative MCl6 rotation, resulting in an extremely low interfacial impedance. A SSSB comprising a NaCrO2 + NYZC composite cathode, Na3PS4 electrolyte, and Na-Sn anode exhibits an exceptional first-cycle Coulombic efficiency of 97.1% at room temperature and can cycle over 1000 cycles with 89.3% capacity retention at 40 °C. These findings highlight the immense potential of halides for SSSB applications.
Conflict of interest statement
R.S. and G.V. are employees of Shell International Exploration and Production Inc., USA and Shell Global Solutions International BV, Netherlands, respectively. A patent was filed for this work through the UCSD Office of Innovation and Commercialization. The remaining authors declare no competing interests.
Figures




References
-
- Richards WD, Miara LJ, Wang Y, Kim JC, Ceder G. Interface Stability in Solid-State Batteries. Chem. Mater. 2016;28:266–273. doi: 10.1021/acs.chemmater.5b04082. - DOI
-
- Kato Y, et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy. 2016;1:1–7. doi: 10.1038/nenergy.2016.30. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

