Screening of metabolic modulators identifies new strategies to target metabolic reprogramming in melanoma
- PMID: 33623106
- PMCID: PMC7902673
- DOI: 10.1038/s41598-021-83796-8
Screening of metabolic modulators identifies new strategies to target metabolic reprogramming in melanoma
Abstract
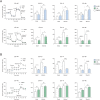
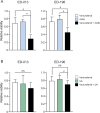
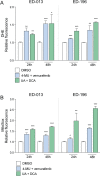
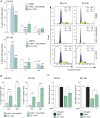
The prognosis of metastatic melanoma remains poor due to de novo or acquired resistance to immune and targeted therapies. Previous studies have shown that melanoma cells have perturbed metabolism and that cellular metabolic pathways represent potential therapeutic targets. To support the discovery of new drug candidates for melanoma, we examined 180 metabolic modulators, including phytochemicals and anti-diabetic compounds, for their growth-inhibitory activities against melanoma cells, alone and in combination with the BRAF inhibitor vemurafenib. Two positive hits from this screen, 4-methylumbelliferone (4-MU) and ursolic acid (UA), were subjected to validation and further characterization. Metabolic analysis showed that 4-MU affected cellular metabolism through inhibition of glycolysis and enhanced the effect of vemurafenib to reduce the growth of melanoma cells. In contrast, UA reduced mitochondrial respiration, accompanied by an increase in the glycolytic rate. This metabolic switch potentiated the growth-inhibitory effect of the pyruvate dehydrogenase kinase inhibitor dichloroacetate. Both drug combinations led to increased production of reactive oxygen species, suggesting the involvement of oxidative stress in the cellular response. These results support the potential use of metabolic modulators for combination therapies in cancer and may encourage preclinical validation and clinical testing of such treatment strategies in patients with metastatic melanoma.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

