FLT3 inhibitor lestaurtinib plus chemotherapy for newly diagnosed KMT2A-rearranged infant acute lymphoblastic leukemia: Children's Oncology Group trial AALL0631
- PMID: 33623141
- PMCID: PMC8763141
- DOI: 10.1038/s41375-021-01177-6
FLT3 inhibitor lestaurtinib plus chemotherapy for newly diagnosed KMT2A-rearranged infant acute lymphoblastic leukemia: Children's Oncology Group trial AALL0631
Erratum in
-
Correction to: FLT3 inhibitor lestaurtinib plus chemotherapy for newly diagnosed KMT2A-rearranged infant acute lymphoblastic leukemia: Children's Oncology Group trial AALL0631.Leukemia. 2021 May;35(5):1527. doi: 10.1038/s41375-021-01245-x. Leukemia. 2021. PMID: 33846544 No abstract available.
Abstract
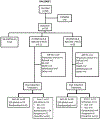
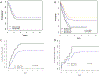
Infants with KMT2A-rearranged acute lymphoblastic leukemia (KMT2A-r ALL) have a poor prognosis. KMT2A-r ALL overexpresses FLT3, and the FLT3 inhibitor (FLT3i) lestaurtinib potentiates chemotherapy-induced cytotoxicity in preclinical models. Children's Oncology Group (COG) AALL0631 tested whether adding lestaurtinib to post-induction chemotherapy improved event-free survival (EFS). After chemotherapy induction, KMT2A-r infants received either chemotherapy only or chemotherapy plus lestaurtinib. Correlative assays included FLT3i plasma pharmacodynamics (PD), which categorized patients as inhibited or uninhibited, and FLT3i ex vivo sensitivity (EVS), which categorized leukemic blasts as sensitive or resistant. There was no difference in 3-year EFS between patients treated with chemotherapy plus lestaurtinib (n = 67, 36 ± 6%) vs. chemotherapy only (n = 54, 39 ± 7%, p = 0.67). However, for the lestaurtinib-treated patients, FLT3i PD and FLT3i EVS significantly correlated with EFS. For FLT3i PD, EFS for inhibited/uninhibited was 59 ± 10%/28 ± 7% (p = 0.009) and for FLTi EVS, EFS for sensitive/resistant was 52 ± 8%/5 ± 5% (p < 0.001). Seventeen patients were both inhibited and sensitive, with an EFS of 88 ± 8%. Adding lestaurtinib did not improve EFS overall, but patients achieving potent FLT3 inhibition and those whose leukemia blasts were sensitive FLT3-inhibition ex vivo did benefit from the addition of lestaurtinib. Patient selection and PD-guided dose escalation may enhance the efficacy of FLT3 inhibition for KMT2A-r infant ALL.
Figures
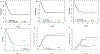

References
-
- Brown P, Pieters R, Biondi A. How I treat infant leukemia. Blood. 2019;133:205–14. - PubMed
-
- Pieters R, Schrappe M, De Lorenzo P, Hann I, De Rossi G, Felice M, et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet. 2007;370: 240–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

