In vitro Study on Synergistic Interactions Between Free and Encapsulated Q-Griffithsin and Antiretrovirals Against HIV-1 Infection
- PMID: 33623382
- PMCID: PMC7894819
- DOI: 10.2147/IJN.S287310
In vitro Study on Synergistic Interactions Between Free and Encapsulated Q-Griffithsin and Antiretrovirals Against HIV-1 Infection
Abstract
Introduction: Human immunodeficiency virus (HIV) remains a persistent global challenge, impacting 38 million people worldwide. Antiretrovirals (ARVs) including tenofovir (TFV), raltegravir (RAL), and dapivirine (DAP) have been developed to prevent and treat HIV-1 via different mechanisms of action. In parallel, a promising biological candidate, griffithsin (GRFT), has demonstrated outstanding preclinical safety and potency against HIV-1. While ARV co-administration has been shown to enhance virus inhibition, synergistic interactions between ARVs and the oxidation-resistant variant of GRFT (Q-GRFT) have not yet been explored. Here, we co-administered Q-GRFT with TFV, RAL, and DAP, in free and encapsulated forms, to identify unique protein-drug synergies.
Methods: Nanoparticles (NPs) were synthesized using a single or double-emulsion technique and release from each formulation was assessed in simulated vaginal fluid. Next, each ARV, in free and encapsulated forms, was co-administered with Q-GRFT or Q-GRFT NPs to evaluate the impact of co-administration in HIV-1 pseudovirus assays, and the combination indices were calculated to identify synergistic interactions. Using the most synergistic formulations, we investigated the effect of agent incorporation in NP-fiber composites on release properties. Finally, NP safety was assessed in vitro using MTT assay.
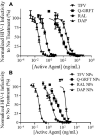
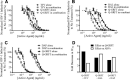
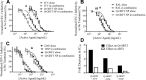
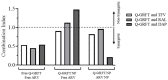
Results: All active agents were encapsulated in NPs with desirable encapsulation efficiency (15-100%), providing ~20% release over 2 weeks. The co-administration of free Q-GRFT with each free ARV resulted in strong synergistic interactions, relative to each agent alone. Similarly, Q-GRFT NP and ARV NP co-administration resulted in synergy across all formulations, with the most potent interactions between encapsulated Q-GRFT and DAP. Furthermore, the incorporation of Q-GRFT and DAP in NP-fiber composites resulted in burst release of DAP and Q-GRFT with a second phase of Q-GRFT release. Finally, all NP formulations exhibited safety in vitro.
Conclusions: This work suggests that Q-GRFT and ARV co-administration in free or encapsulated forms may improve efficacy in achieving prophylaxis.
Keywords: HIV-1 prevention; antiretrovirals; electrospun fibers; griffithsin; microbicide; nanoparticles; synergy.
© 2021 Minooei et al.
Conflict of interest statement
The authors declare they have no competing interests.
Figures




Similar articles
-
Drug synergy of tenofovir and nanoparticle-based antiretrovirals for HIV prophylaxis.PLoS One. 2013 Apr 22;8(4):e61416. doi: 10.1371/journal.pone.0061416. Print 2013. PLoS One. 2013. PMID: 23630586 Free PMC article.
-
pH-responsive delivery of Griffithsin from electrospun fibers.Eur J Pharm Biopharm. 2019 May;138:64-74. doi: 10.1016/j.ejpb.2018.04.013. Epub 2018 Apr 23. Eur J Pharm Biopharm. 2019. PMID: 29698714
-
Griffithsin-Modified Electrospun Fibers as a Delivery Scaffold To Prevent HIV Infection.Antimicrob Agents Chemother. 2016 Oct 21;60(11):6518-6531. doi: 10.1128/AAC.00956-16. Print 2016 Nov. Antimicrob Agents Chemother. 2016. PMID: 27550363 Free PMC article.
-
Clinical development of microbicides for the prevention of HIV infection.Curr Pharm Des. 2004;10(3):315-36. doi: 10.2174/1381612043386374. Curr Pharm Des. 2004. PMID: 14754390 Review.
-
Stampidine as a promising antiretroviral drug candidate for pre-exposure prophylaxis against sexually transmitted HIV/AIDS.Expert Opin Investig Drugs. 2012 Apr;21(4):489-500. doi: 10.1517/13543784.2012.664635. Epub 2012 Feb 24. Expert Opin Investig Drugs. 2012. PMID: 22360744 Review.
Cited by
-
The Advances of Broad-Spectrum and Hot Anti-Coronavirus Drugs.Microorganisms. 2022 Jun 26;10(7):1294. doi: 10.3390/microorganisms10071294. Microorganisms. 2022. PMID: 35889013 Free PMC article. Review.
-
Natural Products with Inhibitory Activity against Human Immunodeficiency Virus Type 1.Adv Virol. 2021 May 29;2021:5552088. doi: 10.1155/2021/5552088. eCollection 2021. Adv Virol. 2021. PMID: 34194504 Free PMC article. Review.
-
Comprehensive analysis of lectin-glycan interactions reveals determinants of lectin specificity.PLoS Comput Biol. 2021 Oct 6;17(10):e1009470. doi: 10.1371/journal.pcbi.1009470. eCollection 2021 Oct. PLoS Comput Biol. 2021. PMID: 34613971 Free PMC article.
-
Sustained Delivery of the Antiviral Protein Griffithsin and Its Adhesion to a Biological Surface by a Silk Fibroin Scaffold.Materials (Basel). 2023 Aug 9;16(16):5547. doi: 10.3390/ma16165547. Materials (Basel). 2023. PMID: 37629837 Free PMC article.
References
-
- Gama L, Koup RA. New-generation high-potency and designer antibodies: role in HIV-1 treatment. Annu Rev Med. 2018;69:409–419. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

