Overexpression of miR125b Promotes Osteoporosis Through miR-125b-TRAF6 Pathway in Postmenopausal Ovariectomized Rats
- PMID: 33623402
- PMCID: PMC7894909
- DOI: 10.2147/DMSO.S288338
Overexpression of miR125b Promotes Osteoporosis Through miR-125b-TRAF6 Pathway in Postmenopausal Ovariectomized Rats
Abstract
Background: Postmenopausal osteoporosis is one of the most common types of osteoporosis that women suffer from. Studies involving molecular mechanisms for designing better therapeutic strategies for postmenopausal osteoporosis are still rare. The present study investigates the role of miR-125b in postmenopausal osteoporosis.
Methods: Microarray analysis was done to screen the gene database. Tissue samples of postmenopausal women were collected to study the miRNA profiles. MC3T3-E1 cells were used and were submitted for transfection. CCK-8 assay was done to check the viability of cells, whereas toxicity was done by lactate dehydrogenase assay kit. TargetScan was done to target genes of miR-125b followed by confirmation by Luciferase reporter assay. For animal studies a rat model of ovariectomized rats was created. Bone mineral density and biomechanics were measured by densitometer. The mRNA levels were assessed by qRT-PCR and proteins by Western blot assay.
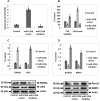
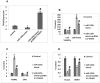
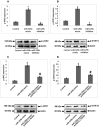
Results: miR-125b was over-expressed in human osteoporosis samples. In vitro studies suggested that miR-125b suppressed the cell viability and promoted release of LDH, it also enhanced the RANKL/OPG ratio and suppressed levels of BMP2 and Runx2. Bioinformatics identified TRAF6 as a potential target of miR-125b, further confirmed by luciferase assay, also miR-125b negatively regulated the levels of TRAF6 gene in osteoporosis bones involving the JAK2/STAT3 cascade. In the rat model, miR-125b decreased the bone mineral density and biomechanical parameters in bones by altering the TRAF6 gene involving the JAK2/STAT3 pathway.
Conclusion: The outcomes suggested that miR-125b was responsible for the development of postmenopausal osteoporosis and promoted its progression by the TRAF6 gene via the JAK2/STAT3 pathway.
Keywords: JAK2/STAT3 pathway; TRAF6; miR‐125b; osteoporosis.
© 2021 Wang et al.
Conflict of interest statement
The authors declare no conflicts of interest for this work.
Figures




References
-
- Chen X, Zhang S, Chen X, et al. Emodin promotes the osteogenesis of MC3T3‐E1 cells via BMP‐9/Smad pathway and exerts a preventive effect in ovariectomized rats. Acta Biochimica et Biophysica Sinica. 2017;49:867–878. - PubMed
-
- Eastell R, O’Neill T, Hofbauer L, et al. Postmenopausal osteoporosis. Nat Rev Dis Primers. 2016;2:1–16. - PubMed
-
- Black DM, Rosen CJ. Postmenopausal osteoporosis. N Engl J Med. 2016;374:254‐262. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

