Effect of Cisplatin Arterial Infusion (CAI) on Primary Nonmetastatic Pelvic Osteosarcoma: A Preliminary Study
- PMID: 33623429
- PMCID: PMC7894794
- DOI: 10.2147/CMAR.S294677
Effect of Cisplatin Arterial Infusion (CAI) on Primary Nonmetastatic Pelvic Osteosarcoma: A Preliminary Study
Abstract
Purpose: The critical role of arterial infusion chemotherapy in the multimodal treatment of extremity bone cancer has been investigated extensively, but few studies have focused on pelvic osteosarcoma. Therefore, we attempted to evaluate the clinical significance of arterial infusion chemotherapy in the treatment of pelvic osteosarcoma.
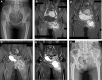
Patients and methods: We combined a cisplatin arterial infusion regimen with multidrug systematic chemotherapy as a neoadjuvant protocol for the treatment of pelvic osteosarcoma. The course number and dosage of cisplatin arterial infusion were adjusted to achieve a maximal tumor response evaluated by contrast-enhanced MRI per RECIST 1.1. Good responders received the same systematic combination for postoperative chemotherapy, and poor responders received second-line therapy. Twelve patients with nonmetastatic high-grade pelvic osteosarcoma were included. Survival, chemotherapy response and adverse events data were analyzed.
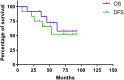
Results: The mean follow-up period was 56.1 months. Four patients died of refractory tumor progression, and 1 patient with local recurrence had no evidence of disease for 27 months after receiving secondary amputation and resection. Kaplan-Meier survival analysis demonstrated a 57.8% overall survival and 52.5% event-free survival rate at 5 years. Eight of 12 patients had a >90% tumor necrosis rate according to histopathologic examinations. The rates of local adverse events were lower than those reported for extremity osteosarcoma.
Conclusion: Our study initially indicated that the cisplatin arterial infusion regimen was a potential therapy with good tolerance in the treatment of pelvic osteosarcoma.
Keywords: arterial infusion; chemotherapy toxicity; cisplatin; osteosarcoma; pelvis.
© 2021 Hu et al.
Conflict of interest statement
No commercial sponsorship was involved in any part of this study. The authors report no conflicts of interest related to this work.
Figures

Similar articles
-
Neoadjuvant Chemotherapy Followed by Delayed Surgery: Is it Necessary for All Patients With Nonmetastatic High-Grade Pelvic Osteosarcoma?Clin Orthop Relat Res. 2018 Nov;476(11):2177-2186. doi: 10.1097/CORR.0000000000000387. Clin Orthop Relat Res. 2018. PMID: 29912746 Free PMC article.
-
Intraarterial Cisplatin and intravenous adriamycin in nonmetastatic osteosarcoma of the extremities: a single institution experience in Taiwan.Chang Gung Med J. 2009 Jan-Feb;32(1):72-80. Chang Gung Med J. 2009. PMID: 19292942
-
Superior survival in treatment of primary nonmetastatic pediatric osteosarcoma of the extremity.Ann Surg Oncol. 2003 Jun;10(5):498-507. doi: 10.1245/aso.2003.03.061. Ann Surg Oncol. 2003. PMID: 12794015 Clinical Trial.
-
Childhood osteosarcoma: multimodal therapy in a single-institution Turkish series.Cancer Treat Res. 2009;152:319-38. doi: 10.1007/978-1-4419-0284-9_17. Cancer Treat Res. 2009. PMID: 20213399 Review.
-
The treatment of nonmetastatic high grade osteosarcoma of the extremity: review of the Italian Rizzoli experience. Impact on the future.Cancer Treat Res. 2009;152:275-87. doi: 10.1007/978-1-4419-0284-9_14. Cancer Treat Res. 2009. PMID: 20213396 Review.
Cited by
-
Radiomics analysis in differentiating osteosarcoma and chondrosarcoma based on T2-weighted imaging and contrast-enhanced T1-weighted imaging.Sci Rep. 2024 Nov 4;14(1):26594. doi: 10.1038/s41598-024-78245-1. Sci Rep. 2024. PMID: 39496777 Free PMC article.
References
-
- Lagmay JP, Krailo MD, Dang H, et al. Outcome of patients with recurrent osteosarcoma enrolled in seven Phase II trials through children’s cancer group, pediatric oncology group, and children’s oncology group: learning from the past to move forward. J Clin Oncol. 2016;34(25):3031–3038. doi:10.1200/jco.2015.65.5381 - DOI - PMC - PubMed
-
- Pappo AS, Vassal G, Crowley JJ, et al. A Phase 2 trial of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF-1R), in patients with recurrent or refractory rhabdomyosarcoma, osteosarcoma, synovial sarcoma, and other soft tissue sarcomas: results of a sarcoma alliance for research through collaboration study. Cancer. 2014;120(16):2448–2456. doi:10.1002/cncr.28728 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

