Synthesis and Characterization of Silver-Coated Polymeric Scaffolds for Bone Tissue Engineering: Antibacterial and In Vitro Evaluation of Cytotoxicity and Biocompatibility
- PMID: 33623844
- PMCID: PMC7893789
- DOI: 10.1021/acsomega.0c05596
Synthesis and Characterization of Silver-Coated Polymeric Scaffolds for Bone Tissue Engineering: Antibacterial and In Vitro Evaluation of Cytotoxicity and Biocompatibility
Abstract
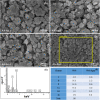
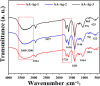
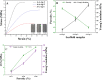
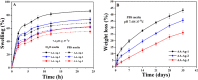
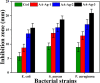
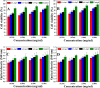
In bone tissue engineering, multifunctional composite materials are very challenging. Bone tissue engineering is an innovative technique to develop biocompatible scaffolds with suitable orthopedic applications with enhanced antibacterial and mechanical properties. This research introduces a polymeric nanocomposite scaffold based on arabinoxylan-co-acrylic acid, nano-hydroxyapatite (nHAp), nano-aluminum oxide (nAl2O3), and graphene oxide (GO) by free-radical polymerization for the development of porous scaffolds using the freeze-drying technique. These polymeric nanocomposite scaffolds were coated with silver (Ag) nanoparticles to improve antibacterial activities. Together, nHAp, nAl2O3, and GO enhance the multifunctional properties of materials, which regulate their physicochemical and biomechanical properties. Results revealed that the Ag-coated polymeric nanocomposite scaffolds had excellent antibacterial properties and better microstructural properties. Regulated morphological properties and maximal antibacterial inhibition zones were found in the porous scaffolds with the increasing amount of GO. Moreover, the nanosystem and the polymeric matrix have improved the compressive strength (18.89 MPa) and Young's modulus (198.61 MPa) of scaffolds upon increasing the amount of GO. The biological activities of the scaffolds were investigated against the mouse preosteoblast cell lines (MC3T3-E1) and increasing the quantities of GO helps cell adherence and proliferation. Therefore, our findings showed that these silver-coated polymeric nanocomposite scaffolds have the potential for engineering bone tissue.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Rosellini E.; Zhang Y. S.; Migliori B.; Barbani N.; Lazzeri L.; Shin S. R.; Dokmeci M. R.; Cascone M. G. Protein/polysaccharide-based scaffolds mimicking native extracellular matrix for cardiac tissue engineering applications. J. Biomed. Mater. Res., Part A 2018, 106, 769–781. 10.1002/jbm.a.36272. - DOI - PMC - PubMed
-
- Bassas-Galia M.; Follonier S.; Pusnik M.; Zinn M.. Natural Polymers: A Source of Inspiration. In Bioresorbable polymers for biomedical applications, From Fundamentals to Translational Medicine; Perale G., Hilborn J., Eds.; Woodhead Publishing, 2017; pp 31–64.
-
- Kiran A. S. K.; Sampath Kumar T. S.; Perumal G.; Sanghavi R.; Doble M.; Ramakrishna S. Dual nanofibrous bioactive coating and antimicrobial surface treatment for infection resistant titanium implants. Prog. Org. Coat. 2018, 121, 112–119. 10.1016/j.porgcoat.2018.04.028. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

