(-)-Epigallocatechin-3-gallate Inhibits Human and Rat Renal Organic Anion Transporters
- PMID: 33623845
- PMCID: PMC7893792
- DOI: 10.1021/acsomega.0c05586
(-)-Epigallocatechin-3-gallate Inhibits Human and Rat Renal Organic Anion Transporters
Abstract
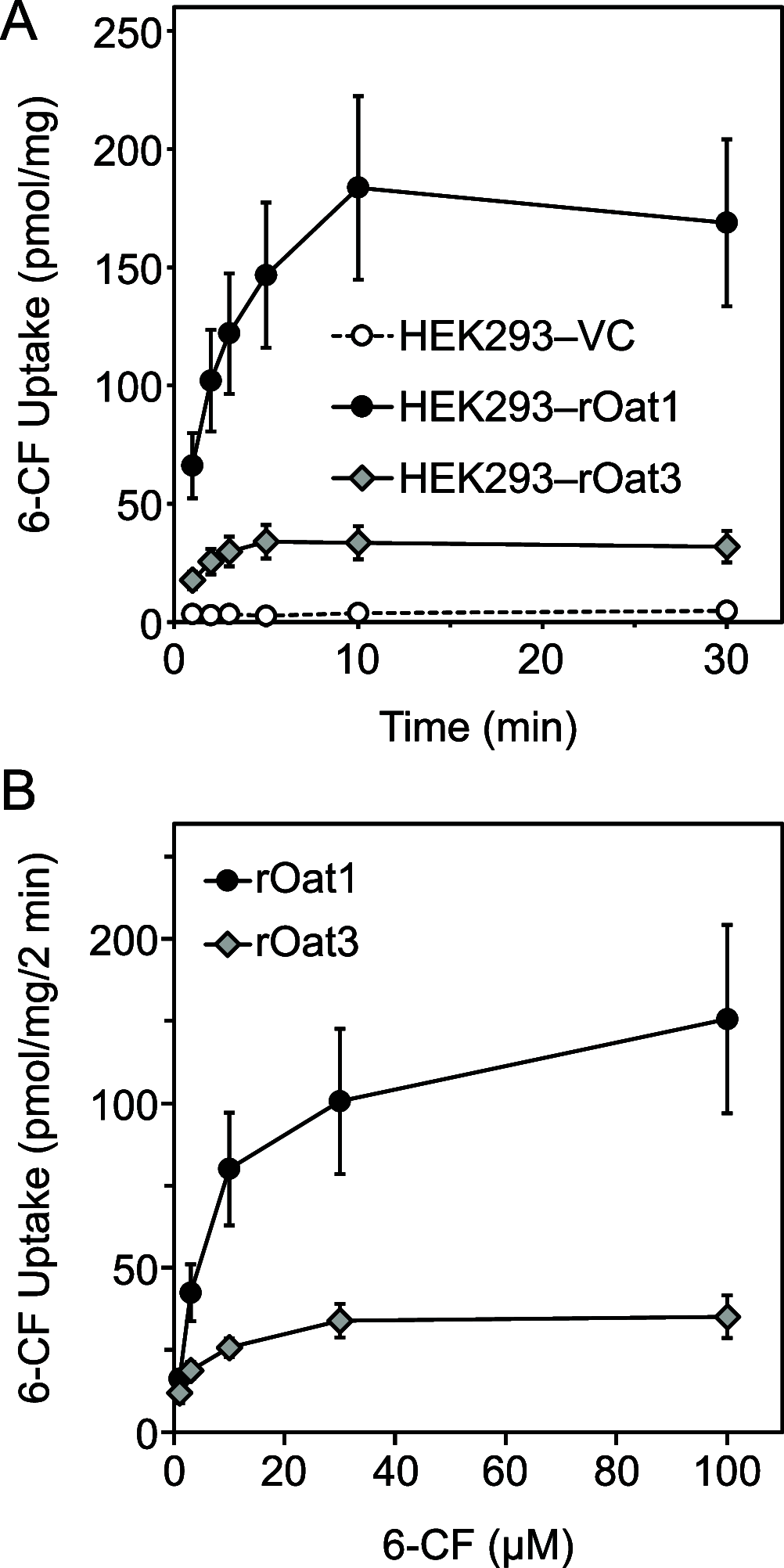
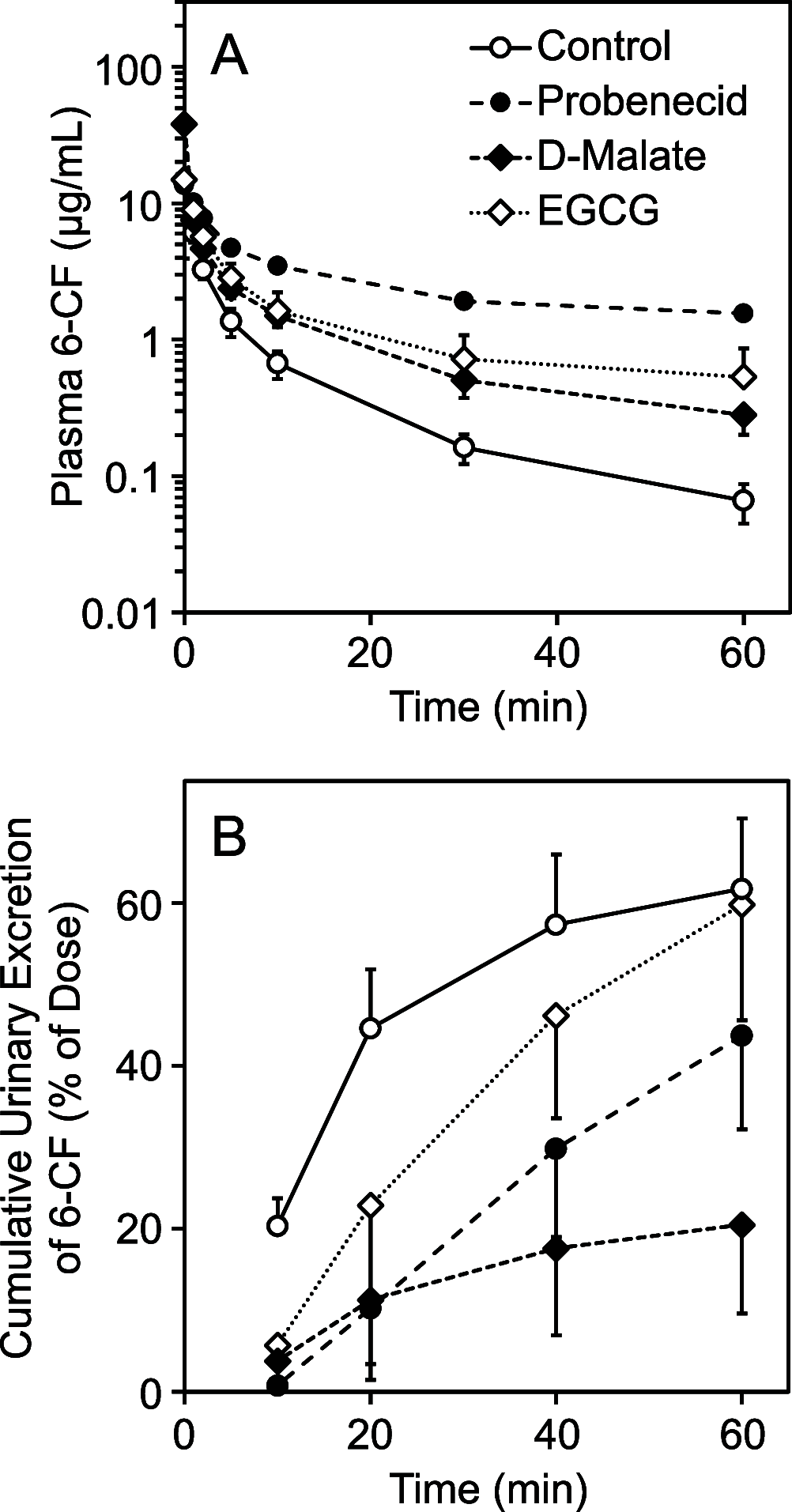
Organic anion transporter 1 (OAT1, SLC22A6) and 3 (OAT3, SLC22A8) are multispecific drug transporters highly expressed on the basolateral membranes of the renal proximal tubules. OAT1 and OAT3 mediate the tubular secretion of clinically significant drugs; thus, they influence the pharmacokinetics of drugs and further determine their efficacy and toxicity. OAT1 and OAT3 are also the target of drug-drug interactions. In this study, we examined the effects of the tea catechin (-)-epigallocatechin-3-gallate (EGCG) on human (h) and rat (r) OAT1 and OAT3 using the fluorescent organic anion 6-carboxyfluorescein (6-CF) and hOAT1-, hOAT3-, rOat1-, or rOat3-expressing HEK293 cells and on renal elimination of 6-CF in rats. 6-CF is transported by hOAT1, hOAT3, rOat1, and rOat3. 6-CF is urinary excreted by Oats in rats. EGCG, a dominant catechin in green tea leaf, inhibits human and rat OAT1 and OAT3 and reduces the renal elimination of 6-CF in rats. Our findings are useful for the assessment of food-drug interactions mediated by renal OATs.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Characterization of uremic toxin transport by organic anion transporters in the kidney.Kidney Int. 2004 Jan;65(1):162-74. doi: 10.1111/j.1523-1755.2004.00354.x. Kidney Int. 2004. PMID: 14675047
-
Transport of Kynurenic Acid by Rat Organic Anion Transporters rOAT1 and rOAT3: Species Difference between Human and Rat in OAT1.Int J Tryptophan Res. 2013;6:1-6. doi: 10.4137/IJTR.S11206. Epub 2013 Feb 17. Int J Tryptophan Res. 2013. PMID: 23467467 Free PMC article.
-
Regulation of renal organic anion transporter 3 (SLC22A8) expression and function by the integrity of lipid raft domains and their associated cytoskeleton.Cell Physiol Biochem. 2013;31(4-5):565-78. doi: 10.1159/000350077. Epub 2013 Apr 26. Cell Physiol Biochem. 2013. PMID: 23615001 Free PMC article.
-
Roles of organic anion transporters (OATs) in renal proximal tubules and their localization.Anat Sci Int. 2017 Mar;92(2):200-206. doi: 10.1007/s12565-016-0369-3. Epub 2016 Sep 10. Anat Sci Int. 2017. PMID: 27614971 Review.
-
Identification and Quantitative Assessment of Uremic Solutes as Inhibitors of Renal Organic Anion Transporters, OAT1 and OAT3.Mol Pharm. 2016 Sep 6;13(9):3130-40. doi: 10.1021/acs.molpharmaceut.6b00332. Epub 2016 Aug 9. Mol Pharm. 2016. PMID: 27467266 Review.
Cited by
-
Recent Advances in Synthetic Drugs and Natural Actives Interacting with OAT3.Molecules. 2023 Jun 13;28(12):4740. doi: 10.3390/molecules28124740. Molecules. 2023. PMID: 37375294 Free PMC article. Review.
-
Effects of aqueous extract from Baiyedancong-Oolong tea on cytochrome P450 enzymes activities, P-gp and OATs transport abilities and transcription levels in mice.Front Nutr. 2023 May 9;10:1136329. doi: 10.3389/fnut.2023.1136329. eCollection 2023. Front Nutr. 2023. PMID: 37229476 Free PMC article.
-
In Vivo Regulation of Small Molecule Natural Products, Antioxidants, and Nutrients by OAT1 and OAT3.Nutrients. 2024 Jul 12;16(14):2242. doi: 10.3390/nu16142242. Nutrients. 2024. PMID: 39064685 Free PMC article.
-
The Use of an Antioxidant Enables Accurate Evaluation of the Interaction of Curcumin on Organic Anion-Transporting Polypeptides 4C1 by Preventing Auto-Oxidation.Int J Mol Sci. 2024 Jan 12;25(2):991. doi: 10.3390/ijms25020991. Int J Mol Sci. 2024. PMID: 38256064 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

