Current Research Trends and Perspectives on Solid-State Nanomaterials in Hydrogen Storage
- PMID: 33623916
- PMCID: PMC7877397
- DOI: 10.34133/2021/3750689
Current Research Trends and Perspectives on Solid-State Nanomaterials in Hydrogen Storage
Abstract
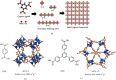
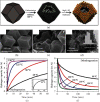
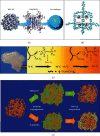
Hydrogen energy, with environment amicable, renewable, efficiency, and cost-effective advantages, is the future mainstream substitution of fossil-based fuel. However, the extremely low volumetric density gives rise to the main challenge in hydrogen storage, and therefore, exploring effective storage techniques is key hurdles that need to be crossed to accomplish the sustainable hydrogen economy. Hydrogen physically or chemically stored into nanomaterials in the solid-state is a desirable prospect for effective large-scale hydrogen storage, which has exhibited great potentials for applications in both reversible onboard storage and regenerable off-board storage applications. Its attractive points include safe, compact, light, reversibility, and efficiently produce sufficient pure hydrogen fuel under the mild condition. This review comprehensively gathers the state-of-art solid-state hydrogen storage technologies using nanostructured materials, involving nanoporous carbon materials, metal-organic frameworks, covalent organic frameworks, porous aromatic frameworks, nanoporous organic polymers, and nanoscale hydrides. It describes significant advances achieved so far, and main barriers need to be surmounted to approach practical applications, as well as offers a perspective for sustainable energy research.
Copyright © 2021 Jie Zheng et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

















References
-
- Smil V. Energy In World History. Abingdon, UK: Routledge; 2019. - DOI
-
- Rhodes R. Energy:A Human History. New York, USA: Simon and Schuster; 2019.
-
- Letcher T. M. Future Energy: Improved, Sustainable and Clean Options for Our Planet. Amsterdam, Netherlands: Elsevier; 2020.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

