Cardiac small-conductance calcium-activated potassium channels in health and disease
- PMID: 33624131
- PMCID: PMC7940285
- DOI: 10.1007/s00424-021-02535-0
Cardiac small-conductance calcium-activated potassium channels in health and disease
Abstract
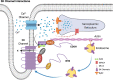
Small-conductance Ca2+-activated K+ (SK, KCa2) channels are encoded by KCNN genes, including KCNN1, 2, and 3. The channels play critical roles in the regulation of cardiac excitability and are gated solely by beat-to-beat changes in intracellular Ca2+. The family of SK channels consists of three members with differential sensitivity to apamin. All three isoforms are expressed in human hearts. Studies over the past two decades have provided evidence to substantiate the pivotal roles of SK channels, not only in healthy heart but also with diseases including atrial fibrillation (AF), ventricular arrhythmia, and heart failure (HF). SK channels are prominently expressed in atrial myocytes and pacemaking cells, compared to ventricular cells. However, the channels are significantly upregulated in ventricular myocytes in HF and pulmonary veins in AF models. Interests in cardiac SK channels are further fueled by recent studies suggesting the possible roles of SK channels in human AF. Therefore, SK channel may represent a novel therapeutic target for atrial arrhythmias. Furthermore, SK channel function is significantly altered by human calmodulin (CaM) mutations, linked to life-threatening arrhythmia syndromes. The current review will summarize recent progress in our understanding of cardiac SK channels and the roles of SK channels in the heart in health and disease.
Keywords: Apamin; Atrial fibrillation; Calcium; Calmodulin; Cardiac action potential; Cardiac arrhythmia; Cardiac repolarization; Heart failure; Pacemaking cell; Pulmonary vein; SK channel; Small-conductance Ca2+-activated K+ channel.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

