Reldesemtiv in Patients with Spinal Muscular Atrophy: a Phase 2 Hypothesis-Generating Study
- PMID: 33624184
- PMCID: PMC8423982
- DOI: 10.1007/s13311-020-01004-3
Reldesemtiv in Patients with Spinal Muscular Atrophy: a Phase 2 Hypothesis-Generating Study
Erratum in
-
Correction to: Reldesemtiv in Patients with Spinal Muscular Atrophy: a Phase 2 Hypothesis-Generating Study.Neurotherapeutics. 2021 Jul;18(3):2130. doi: 10.1007/s13311-021-01120-8. Neurotherapeutics. 2021. PMID: 34731415 Free PMC article. No abstract available.
Abstract
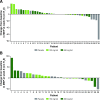
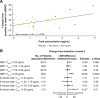
This phase 2, double-blind, placebo-controlled, hypothesis-generating study evaluated the effects of oral reldesemtiv, a fast skeletal muscle troponin activator, in patients with spinal muscular atrophy (SMA). Patients ≥ 12 years of age with type II, III, or IV SMA were randomized into 2 sequential, ascending reldesemtiv dosing cohorts (cohort 1: 150 mg bid or placebo [2:1]; cohort 2: 450 mg bid or placebo [2:1]). The primary objective was to determine potential pharmacodynamic effects of reldesemtiv on 8 outcome measures in SMA, including 6-minute walk distance (6MWD) and maximum expiratory pressure (MEP). Changes from baseline to weeks 4 and 8 were determined. Pharmacokinetics and safety were also evaluated. Patients were randomized to reldesemtiv 150 mg, 450 mg, or placebo (24, 20, and 26, respectively). The change from baseline in 6MWD was greater for reldesemtiv 450 mg than for placebo at weeks 4 and 8 (least squares [LS] mean difference, 35.6 m [p = 0.0037] and 24.9 m [p = 0.058], respectively). Changes from baseline in MEP at week 8 on reldesemtiv 150 and 450 mg were significantly greater than those on placebo (LS mean differences, 11.7 [p = 0.038] and 13.2 cm H2O [p = 0.03], respectively). For 6MWD and MEP, significant changes from placebo were seen in the highest reldesemtiv peak plasma concentration quartile (Cmax > 3.29 μg/mL; LS mean differences, 43.3 m [p = 0.010] and 28.8 cm H2O [p = 0.0002], respectively). Both dose levels of reldesemtiv were well tolerated. Results suggest reldesemtiv may offer clinical benefit and support evaluation in larger SMA patient populations.
Keywords: Reldesemtiv; pharmacodynamics; pharmacokinetics; six-minute walk test; spinal muscular atrophy clinical trial.
© 2021. The Author(s).
Figures



References
-
- Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med 2017;377:1723–1732. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

