Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19
- PMID: 33624299
- PMCID: PMC8094389
- DOI: 10.1002/14651858.CD013587.pub2
Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19
Abstract
Background: The coronavirus disease 2019 (COVID-19) pandemic has resulted in substantial mortality. Some specialists proposed chloroquine (CQ) and hydroxychloroquine (HCQ) for treating or preventing the disease. The efficacy and safety of these drugs have been assessed in randomized controlled trials.
Objectives: To evaluate the effects of chloroquine (CQ) or hydroxychloroquine (HCQ) for 1) treating people with COVID-19 on death and time to clearance of the virus; 2) preventing infection in people at risk of SARS-CoV-2 exposure; 3) preventing infection in people exposed to SARS-CoV-2.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, Current Controlled Trials (www.controlled-trials.com), and the COVID-19-specific resources www.covid-nma.com and covid-19.cochrane.org, for studies of any publication status and in any language. We performed all searches up to 15 September 2020. We contacted researchers to identify unpublished and ongoing studies.
Selection criteria: We included randomized controlled trials (RCTs) testing chloroquine or hydroxychloroquine in people with COVID-19, people at risk of COVID-19 exposure, and people exposed to COVID-19. Adverse events (any, serious, and QT-interval prolongation on electrocardiogram) were also extracted.
Data collection and analysis: Two review authors independently assessed eligibility of search results, extracted data from the included studies, and assessed risk of bias using the Cochrane 'Risk of bias' tool. We contacted study authors for clarification and additional data for some studies. We used risk ratios (RR) for dichotomous outcomes and mean differences (MD) for continuous outcomes, with 95% confidence intervals (CIs). We performed meta-analysis using a random-effects model for outcomes where pooling of effect estimates was appropriate.
Main results: 1. Treatment of COVID-19 disease We included 12 trials involving 8569 participants, all of whom were adults. Studies were from China (4); Brazil, Egypt, Iran, Spain, Taiwan, the UK, and North America (each 1 study); and a global study in 30 countries (1 study). Nine were in hospitalized patients, and three from ambulatory care. Disease severity, prevalence of comorbidities, and use of co-interventions varied substantially between trials. We found potential risks of bias across all domains for several trials. Nine trials compared HCQ with standard care (7779 participants), and one compared HCQ with placebo (491 participants); dosing schedules varied. HCQ makes little or no difference to death due to any cause (RR 1.09, 95% CI 0.99 to 1.19; 8208 participants; 9 trials; high-certainty evidence). A sensitivity analysis using modified intention-to-treat results from three trials did not influence the pooled effect estimate. HCQ may make little or no difference to the proportion of people having negative PCR for SARS-CoV-2 on respiratory samples at day 14 from enrolment (RR 1.00, 95% CI 0.91 to 1.10; 213 participants; 3 trials; low-certainty evidence). HCQ probably results in little to no difference in progression to mechanical ventilation (RR 1.11, 95% CI 0.91 to 1.37; 4521 participants; 3 trials; moderate-certainty evidence). HCQ probably results in an almost three-fold increased risk of adverse events (RR 2.90, 95% CI 1.49 to 5.64; 1394 participants; 6 trials; moderate-certainty evidence), but may make little or no difference to the risk of serious adverse events (RR 0.82, 95% CI 0.37 to 1.79; 1004 participants; 6 trials; low-certainty evidence). We are very uncertain about the effect of HCQ on time to clinical improvement or risk of prolongation of QT-interval on electrocardiogram (very low-certainty evidence). One trial (22 participants) randomized patients to CQ versus lopinavir/ritonavir, a drug with unknown efficacy against SARS-CoV-2, and did not report any difference for clinical recovery or adverse events. One trial compared HCQ combined with azithromycin against standard care (444 participants). This trial did not detect a difference in death, requirement for mechanical ventilation, length of hospital admission, or serious adverse events. A higher risk of adverse events was reported in the HCQ-and-azithromycin arm; this included QT-interval prolongation, when measured. One trial compared HCQ with febuxostat, another drug with unknown efficacy against SARS-CoV-2 (60 participants). There was no difference detected in risk of hospitalization or change in computed tomography (CT) scan appearance of the lungs; no deaths were reported. 2. Preventing COVID-19 disease in people at risk of exposure to SARS-CoV-2 Ongoing trials are yet to report results for this objective. 3. Preventing COVID-19 disease in people who have been exposed to SARS-CoV-2 One trial (821 participants) compared HCQ with placebo as a prophylactic agent in the USA (around 90% of participants) and Canada. Asymptomatic adults (66% healthcare workers; mean age 40 years; 73% without comorbidity) with a history of exposure to people with confirmed COVID-19 were recruited. We are very uncertain about the effect of HCQ on the primary outcomes, for which few events were reported: 20/821 (2.4%) developed confirmed COVID-19 at 14 days from enrolment, and 2/821 (0.2%) were hospitalized due to COVID-19 (very low-certainty evidence). HCQ probably increases the risk of adverse events compared with placebo (RR 2.39, 95% CI 1.83 to 3.11; 700 participants; 1 trial; moderate-certainty evidence). HCQ may result in little or no difference in serious adverse events (no RR: no participants experienced serious adverse events; low-certainty evidence). One cluster-randomized trial (2525 participants) compared HCQ with standard care for the prevention of COVID-19 in people with a history of exposure to SARS-CoV-2 in Spain. Most participants were working or residing in nursing homes; mean age was 49 years. There was no difference in the risk of symptomatic confirmed COVID-19 or production of antibodies to SARS-CoV-2 between the two study arms.
Authors' conclusions: HCQ for people infected with COVID-19 has little or no effect on the risk of death and probably no effect on progression to mechanical ventilation. Adverse events are tripled compared to placebo, but very few serious adverse events were found. No further trials of hydroxychloroquine or chloroquine for treatment should be carried out. These results make it less likely that the drug is effective in protecting people from infection, although this is not excluded entirely. It is probably sensible to complete trials examining prevention of infection, and ensure these are carried out to a high standard to provide unambiguous results.
Copyright © 2021 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
BS is a Clinical Research Fellow for the National Institute for Health Research (NIHR) Global Health Research Group on Brain Infections at the University of Liverpool (No. 17/63/110) and in the NIHR Health Protection Research Unit on Emerging and Zoonotic Infections, and also works at the Royal Liverpool University Hospital, UK, and Christian Medical College, Vellore, India. He has no known conflicts of interest to declare with respect to chloroquine or hydroxychloroquine for the management of COVID‐19.
HR is a Specialist Registrar in Clinical Pharmacology in Liverpool, and is employed as a full‐time NHS clinician, and has no conflicts of interest to declare with respect to chloroquine or hydroxychloroquine for the management of COVID‐19.
TK has no conflicts of interest to declare with respect to chloroquine or hydroxychloroquine for the management of COVID‐19.
MC has no conflicts of interest to declare with respect to chloroquine or hydroxychloroquine for the management of COVID‐19.
TF has no conflicts of interest to declare with respect to chloroquine or hydroxychloroquine for the management of COVID‐19.
Figures
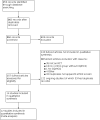
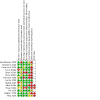






























Update of
- doi: 10.1002/14651858.CD013587
References
References to studies included in this review
Abd‐Elsalam 2020 {published data only}
Boulware 2020 {published data only}
Cavalcanti 2020 {published data only}
Chen 2020a {published data only}
Chen 2020b {published data only}
-
- Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. www.medrxiv.org/content/10.1101/2020.03.22.20040758v2 (accessed 20 April 2020). [DOI: 10.1101/2020.03.22.20040758] - DOI
Chen 2020c {published data only}
-
- Chen CP, Lin YC, Chen TC, Tseng TY, Wong HL, Kuo CY, et al. A multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tolerability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate Coronavirus disease 2019 (COVID-19). www.medrxiv.org/content/10.1101/2020.07.08.20148841v1 (accessed prior to 18 December 2020). [DOI: 10.1101/2020.07.08.20148841] - DOI - PMC - PubMed
Davoodi 2020 {published data only}
Horby 2020 {published data only (unpublished sought but not used)}
-
- Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. www.medrxiv.org/content/10.1101/2020.07.15.20151852v1 (accessed 15 July 2020). [DOI: 10.1101/2020.07.15.20151852] - DOI
Huang 2020 {published data only}
Mitjà 2020a {published data only}
Mitjà 2020b {published data only}
-
- Mitjà O, Ubals M, Corbacho M, Alemany A, Suner C, Tebe C, et al. A cluster-randomized trial of hydroxychloroquine as prevention of Covid-19 transmission and disease. www.medrxiv.org/content/10.1101/2020.07.20.20157651v1 (accessed prior to 18 December 2020). [DOI: 10.1101/2020.07.20.20157651] - DOI
Pan 2020 {published data only (unpublished sought but not used)}
-
- WHO Solidarity trial consortium, Pan H, Peto R, Abdool Karim Q, Karim QA, Alejandria M, et al. Repurposed antiviral drugs for COVID-19 - interim WHO SOLIDARITY trial results. www.medrxiv.org/content/10.1101/2020.10.15.20209817v1 (accessed prior to 18 December 2020). [DOI: 10.1101/2020.10.15.20209817] - DOI - PMC - PubMed
Skipper 2020 {published data only}
References to studies excluded from this review
Agrawal 2020 {published data only}
Alia 2020 {published data only}
Brown 2020 {published data only}
ChiCTR2000029542 {published data only}
-
- ChiCTR2000029542. Study for the efficacy of chloroquine in patients with novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showprojen.aspx?proj=48968 (first received 3 February 2020). [ChiCTR2000029542]
ChiCTR2000029609 {published data only}
-
- ChiCTR2000029609. A prospective, open-label, multiple-center study for the efficacy of chloroquine phosphate in patients with novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showprojen.aspx?proj=49145 (first received 6 February 2020). [ChiCTR2000029609]
ChiCTR2000029898 {published data only}
-
- ChiCTR2000029898. Evaluation the efficacy and safety of hydroxychloroquine sulfate in comparison with phosphate chloroquine in severe patients with novel coronavirus pneumonia (COVID-19): a randomized, open-label, parallel, controlled trial. www.chictr.org.cn/showprojen.aspx?proj=49482 (first received 16 February 2020). [ChiCTR2000029898]
ChiCTR2000029899 {published data only}
-
- ChiCTR2000029899. Evaluation the efficacy and safety of hydroxychloroquine sulfate in comparison with phosphate chloroquine in mild and commen (sic) patients with novel coronavirus pneumonia (COVID-19): a randomized, open-label, parallel, controlled trial. www.chictr.org.cn/showprojen.aspx?proj=49536 (first received 16 February 2020). [ChiCTR2000029899]
Colson 2020a {published data only}
Colson 2020b {published data only}
EUCTR2020‐000890‐25‐FR {published data only}
-
- EUCTR2020-000890-25-FR. Hydroxychloroquine as a treatment for coronavirus disease COVID-19. www.clinicaltrialsregister.eu/ctr-search/search?query=EUCTR2020-000890-2... (first received 10 March 2020). [EUCTR2020-000890-25-FR]
EUCTR2020‐001421‐31‐ES {published data only}
-
- EUCTR2020-001421-31-ES. Clinical trial randomized, unblinded and controled for evaluation of efficacy and safety of hydroxychloroquine chemoprophylaxis against SARS-CoV-2 (COVID-19) infection in healthcare professionals. www.clinicaltrialsregister.eu/ctr-search/search?query=EUCTR2020-001421-3... (first received 7 April 2020). [EUCTR2020-001421-31-ES]
Ferner 2020 {published data only}
Gao 2020 {published data only}
Gendrot 2020 {published data only}
Heldwein 2020 {published data only}
Lee 2020 {published data only}
Lofgren 2020 {published data only}
Nau 2020 {published data only}
-
- Nau JY. Coronavirus epidemic and chloroquine controversy. Revue Medicale Suisse 2020;685:510-1. - PubMed
NCT04304053 {published data only}
-
- NCT04304053. Treatment of COVID-19 cases and chemoprophylaxis of contacts as prevention (HCQ4COV19). clinicaltrials.gov/ct2/show/NCT04304053 (first received 11 March 2020). [NCT04304053]
NCT04321278 {published data only}
-
- NCT04321278. Safety and efficacy of hydroxychloroquine associated with azithromycin in SARS-CoV-2 virus (Coalition Covid-19 Brasil II). clinicaltrials.gov/show/NCT04321278 (first received 25 March 2020). [NCT04321278]
NCT04321993 {published data only}
-
- NCT04321993. Treatment of moderate to severe coronavirus disease (COVID-19) in hospitalized patients. clinicaltrials.gov/show/NCT04321993 (first received 26 March 2020). [NCT04321993]
NCT04323527 {published data only}
-
- NCT04323527. Chloroquine diphosphate for the treatment of severe acute respiratory syndrome secondary to SARS-CoV2 (CloroCOVID19). clinicaltrials.gov/ct2/show/NCT04323527 (first received 26 March 2020). [NCT04323527]
NCT04326725 {published data only}
-
- NCT04326725. Proflaxis using hydroxychloroquine plus vitamins-zinc during COVID-19 pandemia. clinicaltrials.gov/show/NCT04326725 (first received 30 March 2020). [NCT04326725]
NCT04329572 {published data only}
-
- NCT04329572. Efficacy and safety of hydroxychloroquine and azithromycin for the treatment of hospitalized patients with moderate to severe COVID-19. clinicaltrials.gov/show/NCT04329572 (first received 1 April 2020). [NCT04329572]
NCT04329611 {published data only}
-
- NCT04329611. ALBERTA HOPE COVID-19 for the prevention of severe COVID19 disease. clinicaltrials.gov/ct2/show/NCT04329611 (first received 1 April 2020). [NCT04329611]
NCT04332094 {published data only}
-
- NCT04332094. Clinical trial of combined use of hydroxychloroquine, azithromycin, and tocilizumab for the treatment of COVID-19 (TOCOVID). clinicaltrials.gov/show/NCT04332094 (first received 2 April 2020). [NCT04332094]
NCT04333225 {published data only}
-
- NCT04333225. Hydroxychloroquine in the prevention of COVID-19 infection in healthcare workers. clinicaltrials.gov/show/NCT04333225 (first received 3 April 2020). [NCT04333225]
NCT04334512 {published data only}
-
- NCT04334512. A study of quintuple therapy to treat COVID-19 infection. clinicaltrials.gov/show/NCT04334512 (first received 6 April 2020). [NCT04334512]
NCT04335084 {published data only}
-
- NCT04335084. A study of hydroxychloroquine, vitamin C, vitamin D, and zinc for the prevention of COVID-19 infection (HELPCOVID-19). clinicaltrials.gov/show/NCT04335084 (first received 6 April 2020). [NCT04335084]
NCT04341493 {published data only}
-
- NCT04341493. Hydroxychloroquine vs nitazoxanide in patients with COVID-19. clinicaltrials.gov/show/NCT04341493 (first received 10 April 2020). [NCT04341493]
NCT04341727 {published data only}
-
- NCT04341727. Hydroxychloroquine, hydroxychloroquine, azithromycin in the treatment of SARS CoV-2 infection (WU352). clinicaltrials.gov/show/NCT04341727 (first received 10 April 2020). [NCT04341727]
NCT04343092 {published data only}
-
- NCT04343092. Efficacy of ivermectin as add on therapy in COVID-19 patients. clinicaltrials.gov/show/NCT04343092 (first received 13 April 2020). [NCT04343092]
NCT04343677 {published data only}
-
- NCT04343677. Military COVID-19 hydroxychloroquine pre-exposure and post-exposure prophylaxis study. Unable to access - removed from clinicaltrials.gov website. [NCT04343677]
NCT04344457 {published data only}
-
- NCT04344457. Evaluate the efficacy and safety of oral hydroxychloroquine, indomethacin and zithromax in subjects with mild symptoms of COVID-19. clinicaltrials.gov/ct2/show/NCT04344457 (first received 14 April 2020). [NCT04344457]
NCT04345419 {published data only}
-
- NCT04345419. A real-life experience on treatment of patients with COVID 19. clinicaltrials.gov/show/NCT04345419 (first received 14 April 2020). [NCT04345419]
NCT04345653 {published data only}
-
- NCT04345653. Hydroxychloroquine as chemoprevention for COVID-19 for high risk healthcare workers. clinicaltrials.gov/show/NCT04345653 (first received 14 April 2020). [NCT04345653]
NCT04346147 {published data only}
-
- NCT04346147. Clinical trial to evaluate efficacy of 3 types of treatment in patients with pneumonia by COVID-19 (Covid-19HUF). clinicaltrials.gov/ct2/show/NCT04346147 (first received 15 April 2020). [NCT04346147]
NCT04347798 {published data only}
-
- NCT04347798. IMPACT: IMPact of Antimalarials on Covid-19 Infections in RAPPORT (IMPACT). clinicaltrials.gov/ct2/show/NCT04347798 (first received 15 April 2020). [NCT04347798]
NCT04348474 {published data only}
-
- NCT04348474. Efficacy and safety of hydroxychloroquine and azithromycin for the treatment of ambulatory patients with mild COVID-19. clinicaltrials.gov/ct2/show/NCT04348474 (first received 16 April 2020). [NCT04348474]
NCT04350281 {published data only}
-
- NCT04350281. Double therapy with IFN-beta 1b and hydroxychloroquine. clinicaltrials.gov/show/NCT04350281 (first received 17 April 2020). [NCT04350281]
NCT04350450 {published data only}
-
- NCT04350450. Hydroxychloroquine treatment of healthcare workers with COVID19 illness at Montefiore. clinicaltrials.gov/show/NCT04350450 (first received 17 April 2020). [NCT04350450]
NCT04351620 {published data only}
-
- NCT04351620. High-dose hydroxychloroquine for the treatment of ambulatory patients with mild COVID-19. clinicaltrials.gov/show/NCT04351620 (first received 17 April 2020). [NCT04351620]
NCT04351919 {published data only}
-
- NCT04351919. Assessment of efficacy and safety of HCQ and antibiotics administrated to patients COVID19(+). clinicaltrials.gov/show/NCT04351919 (first received 17 April 2020). [NCT04351919]
NCT04354870 {published data only}
-
- NCT04354870. COVID-19 PrEP HCW HCQ Study. clinicaltrials.gov/ct2/show/NCT04354870 (first received 21 April 2020). [NCT04354870]
NCT04361461 {published data only}
-
- NCT04361461. Use of hydroxychloroquine alone or associated for inpatients with SARS-CoV-2 virus (COVID-19). clinicaltrials.gov/ct2/show/NCT04361461 (first received 24 April 2020). [NCT04361461]
NCT04362189 {published data only}
-
- NCT04362189. Efficacy and safety study of allogeneic HB-adMSCs for the treatment of COVID-19. clinicaltrials.gov/ct2/show/NCT04362189 (first received 24 April 2020). [NCT04362189]
NCT04370262 {published data only}
-
- NCT04370262. Multi-site adaptive trials for COVID-19. clinicaltrials.gov/ct2/show/NCT04370262 (first received 30 April 2020). [NCT04370262]
NCT04395768 {published data only}
-
- NCT04395768. International ALLIANCE study of therapies to prevent progression of COVID-19. clinicaltrials.gov/ct2/show/NCT04395768 (first received 20 May 2020). [NCT04395768]
Pagliano 2020 {published data only}
-
- Pagliano P, Piazza O, De Caro F, Ascione T, Filippelli A. Is hydroxychloroquine a possible postexposure prophylaxis drug to limit the transmission to healthcare workers exposed to coronavirus disease 2019? Clinical Infectious Diseases 2020;71(15):887-8. [DOI: 10.1093/cid/ciaa320] - DOI - PMC - PubMed
Patri 2020 {published data only}
Principi 2020 {published data only}
Rathi 2020 {published data only}
Sahraei 2020 {published data only}
Yu 2020 {published data only}
Additional references
Agarwal 2020
-
- Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P, PLACID Trial Collaborators. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ 2020;371:m3939. [DOI: 10.1136/bmj.m3939] - DOI - PMC - PubMed
Arshad 2020
Bai 2020
Beigel 2020
Ben‐Zvi 2012
Borba 2020
-
- Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Network Open 2020;3(4):e208857. [DOI: 10.1001/jamanetworkopen.2020.8857] - DOI - PubMed
Brescia‐COVID Group 2020
-
- Brescia-COVID Group. Brescia-COVID Respiratory Severity Scale Algorithm. Step-wise management approach to COVID-19 patients based on clinical severity as of March 27, 2020. www.mdcalc.com/brescia-covid-respiratory-severity-scale-bcrss-algorithm (accessed 6 April 2020).
Cai 2020
Cao 2020
Catteau 2020
-
- Catteau L, Dauby N, Montourcy M, Bottieau E, Hautekiet J, Goetghebeur E, et al. Low-dose hydroxychloroquine therapy and mortality in hospitalized patients with COVID-19: a nationwide observational study of 8075 participants. International Journal of Antimicrobial Agents 2020;56(4):106144. [DOI: 10.1016/j.ijantimicag.2020.106144] - DOI - PMC - PubMed
CDC 2020
-
- Centers for Disease Control and Prevention. Interim guidelines for collecting, handling, and testing clinical specimens from persons for Coronavirus Disease 2019 (COVID-19). www.cdc.gov/coronavirus/2019-nCoV/lab/guidelines-clinical-specimens.html (accessed 20 April 2020).
Chandel 2020
-
- Chandel V, Raj S, Rathi B, Kumar D. In silico identification of potent COVID-19 main protease inhibitors from FDA approved antiviral compounds and active phytochemicals through molecular docking: a drug repurposing approach. www.preprints.org/manuscript/202003.0349/v1 (accessed 6 April 2020). [DOI: 10.20944/preprints202003.0349.v1] - DOI
Chatterjee 2020
CNN 2020
-
- Busari S, Adebayo B, CNN. Nigeria records chloroquine poisoning after Trump endorses it for coronavirus treatment. edition.cnn.com/2020/03/23/africa/chloroquine-trump-nigeria-intl/index.html.
Cortegiani 2020
Covidence [Computer program]
-
- Covidence. Version accessed 6 April 2020. Melbourne, Australia: Veritas Health Innovation, Updated on 4 February 2019. Available at covidence.org.
Deeks 2020
Deng 2020
Ebrahim 2013
Ebrahim 2014
-
- Ebrahim S, Johnston BC, Akl EA, Mustafa RA, Sun X, Walter SD, et al. Addressing continuous data measured with different instruments for participants excluded from trial analysis: a guide for systematic reviewers. Journal of Clinical Epidemiology 2014;67(5):560-70. [DOI: 10.1016/j.jclinepi.2013.11.014] - DOI - PubMed
Elavarasi 2020
Epistemonikos 2020
-
- Epistemonikos COVID-19 LOVE Working Group. Antimalarials for the treatment of COVID-19: systematic review - preliminary report. Last update: 31 March 2020. www.epistemonikos.cl/2020/03/27/systematic-review-preliminary-report-ant... (accessed 7 April 2020).
FDA 2020
-
- US Food and Drug Administration. FDA News Release: Coronavirus (COVID-19) Update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine. www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-... (accessed 8 September 2020).
Fiehn 2020
-
- Fiehn C, Ness T, Weseloh C, Specker C, Hadjiski D, Detert J, et al. Safety management in treatment with antimalarials in rheumatology. Interdisciplinary recommendations on the basis of a systematic literature review. Zeitschrift fur Rheumatologie 2020 March 31 [Epub ahead of print]. [DOI: 10.1007/s00393-020-00785-4] - DOI - PubMed
Fiolet 2020
-
- Fiolet T, Guihur A, Rebeaud M, Mulot M, Peiffer-Smadja N, Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of COVID-19 patients: a systematic review and meta-analysis. Clinical Microbiology and Infection 2020;27(1):138-40. [DOI: 10.1016/ j.cmi.2020.08.022] - PMC - PubMed
Gautret 2020a
-
- Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. International Journal of Antimicrobial Agents 2020 March 20 [Epub ahead of print]. [DOI: 10.1016/j.ijantimicag.2020.105949] - DOI - PMC - PubMed
Gautret 2020b
-
- Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Sevestre J, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Medicine and Infectious Disease 2020;34:101663. [DOI: 10.1016/j.tmaid.2020.101663] - DOI - PMC - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 8 Nov 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available from gradepro.org.
Greenhalgh 2020
Guan 2020
Hernandez 2020
-
- Hernandez AV, Roman YM, Pasupuleti V, Barboza JJ, White CM. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19. Living systematic review. Annals of Internal Medicine 2020;173(4):287-96. - PubMed
Higgins 2019
-
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hoffman 2020
ICMR 2020
-
- Indian Council of Medical Research – National Taskforce for COVID-19. Recommendation on the use of hydroxy-chloroquine as prophylaxis for SARS-CoV-2 infection. icmr.nic.in/sites/default/files/upload_documents/HCQ_Recommendation_22Ma... (accessed 6 April 2020).
ISS 2020
-
- Istituto Superiore di Sanità. Sorveglianza integrata COVID-19 in Italia. www.epicentro.iss.it/coronavirus/sars-cov-2-sorveglianza-dati (accessed 6 April 2020).
JHU 2020
Kapoor 2020
-
- Kapoor KM, Kapoor A. Role of chloroquine and hydroxychloroquine in the treatment of COVID-19 infection - a systematic literature review. www.medrxiv.org/content/10.1101/2020.03.24.20042366v1 (accessed 6 April 2020). [DOI: 10.1101/2020.03.24.20042366] - DOI
Kraemer 2020
Lancet 2020
Lewis 2020
-
- Lewis D. Is the coronavirus airborne? Experts can't agree. www.nature.com/articles/d41586-020-00974-w (accessed 6 April 2020). [DOI: 10.1038/d41586-020-00974-w] - DOI - PubMed
Liu 2020a
Liu 2020b
Machiels 2020
-
- Machiels JD, Bleeker-Rovers CP, Ter Heine R, Rahamat-Langendoen J, Mast Q, Ten Oever J, et al. Reply to Gautret et al: hydroxychloroquine sulfate and azithromycin for COVID-19: what is the evidence and what are the risks? International Journal of Antimicrobial Agents 2020;56(1):106056. [DOI: 10.1016/j.ijantimicag.2020.106056] - DOI - PMC - PubMed
Mahévas 2020
McCormack 2020
-
- McCormack J, Lindblad AJ. Hydroxychloroquine with or without azithromycin for COVID-19. gomainpro.ca/tools-for-practice/articles/details/?id=619&page-title=... (accessed 7 April 2020).
Mehra 2020
Mehta 2020
Million 2020
-
- Million M, Lagier JC, Gautret P, Colson P, Fournier PE, Amrane S, et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Travel Medicine and Infectious Disease 2020;35:101738. [DOI: 10.1016/j.tmaid.2020.101738] - DOI - PMC - PubMed
Molina 2020
-
- Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Médecine et Maladies Infectieuses 2020 March 30 [Epub ahead of print]. [DOI: 10.1016/j.medmal.2020.03.006] - DOI - PMC - PubMed
Nguyen 2020
-
- Nguyen LS, Dolladille C, Drici MD, Fenioux C, Alexandre J, Mira JP, et al. Cardiovascular toxicities associated with hydroxychloroquine and azithromycin: an analysis of the World Health Organization Pharmacovigilance Database. Circulation 2020;142(2):303-5. [DOI: 10.1161/CIRCULATIONAHA.120.048238] - DOI - PMC - PubMed
NIH 2020
-
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. www.covid19treatmentguidelines.nih.gov/ (accessed 9 September 2020).
Owens 2020
Pung 2020
REACT 2020
-
- The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020 Sep 2 [Epub ahead of print]. [DOI: 10.1001/jama.2020.17023] - DOI - PMC - PubMed
RevMan Web 2019 [Computer program]
-
- Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019. Available at: revman.cochrane.org.
Robinson 2020
-
- Robinson J. MHRA instructs all UK hydroxychloroquine COVID-19 clinical trials to suspend recruitment. Pharmaceutical Journal 2020;305(7939):online (no pagination). [DOI: 10.1211/PJ.2020.20208075] - DOI
Rothe 2020
Schrezenmeier 2020
Shah 2020
Singh 2020
Spinner 2020
Steinhardt 2011
Stone 2020
van den Borne 1997
-
- den Borne BE, Dijkmans BA, Rooij HH, le Cessie S, Verweij CL. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. Journal of Rheumatology 1997;24(1):55-60. [PMID: ] - PubMed
van Doremalen 2020
Wang 2020a
Wang 2020b
Wang 2020c
WHO 2019
-
- World Health Organization. WHO Model List of Essential Medicines, 21st List. www.who.int/medicines/publications/essentialmedicines/en (accessed 21 April 2020).
WHO 2020a
-
- World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report – 76. www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed 6 April 2020).
WHO 2020b
-
- World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). www.who.int/publications-detail/report-of-the-who-china-joint-mission-on... (accessed 6 April 2020).
WHO 2020c
-
- World Health Organization. Clinical management of COVID-19: interim guidance 27 May 2020. www.who.int/publications/i/item/clinical-management-of-covid-19 (accessed prior to 18 December 2020).
WHO 2020d
-
- World Health Organization. Corticosteroids for COVID-19: Living Guidance. www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1 (accessed prior to 18 December 2020). [WHO REFERENCE: WHO/2019-nCoV/Corticosteroids/2020.1]
WIV‐ISP 2020
-
- WIV-ISP (Scientific Institute of Public Health, Belgium). Interim clinical guidance for adults with suspected or confirmed COVID-19 in Belgium. epidemio.wiv-isp.be/ID/Documents/Covid19/COVID-19_InterimGuidelines_Trea... (accessed 6 April 2020).
Wong 2020
Wu 2020
Yao 2020
-
- Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clinical Infectious Diseases 2020 March 9 [Epub ahead of print]. [DOI: 10.1093/cid/ciaa237] - DOI - PMC - PubMed
Zang 2020
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

