Persistence of Antibodies to Severe Acute Respiratory Syndrome Coronavirus 2 in Relation to Symptoms in a Nationwide Prospective Study
- PMID: 33624751
- PMCID: PMC7929058
- DOI: 10.1093/cid/ciab172
Persistence of Antibodies to Severe Acute Respiratory Syndrome Coronavirus 2 in Relation to Symptoms in a Nationwide Prospective Study
Abstract
Background: Assessing the duration of immunity following infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a first priority to gauge the degree of protection following infection. Such knowledge is lacking, especially in the general population. Here, we studied changes in immunoglobulin isotype seropositivity and immunoglobulin G (IgG) binding strength of SARS-CoV-2-specific serum antibodies up to 7 months following onset of symptoms in a nationwide sample.
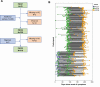
Methods: Participants from a prospective representative serological study in the Netherlands were included based on IgG seroconversion to the spike S1 protein of SARS-CoV-2 (N = 353), with up to 3 consecutive serum samples per seroconverted participant (N = 738). Immunoglobulin M (IgM), immunoglobulin A (IgA), and IgG antibody concentrations to S1, and increase in IgG avidity in relation to time since onset of disease symptoms, were determined.
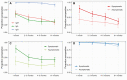
Results: While SARS-CoV-2-specific IgM and IgA antibodies declined rapidly after the first month after disease onset, specific IgG was still present in 92% (95% confidence interval [CI], 89%-95%) of the participants after 7 months. The estimated 2-fold decrease of IgG antibodies was 158 days (95% CI, 136-189 days). Concentrations were sustained better in persons reporting significant symptoms compared to asymptomatic persons or those with mild upper respiratory complaints only. Similarly, avidity of IgG antibodies for symptomatic persons showed a steeper increase over time compared with persons with mild or no symptoms (P = .022).
Conclusions: SARS-CoV-2-specific IgG antibodies persist and show increasing avidity over time, indicative of underlying immune maturation. These data support development of immune memory against SARS-CoV-2, providing insight into protection of the general unvaccinated part of the population.
Clinical trials registration: NL8473 (the Dutch trial registry).
Keywords: COVID-19; avidity/maturation; decay; immunoglobulin G; symptoms.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

Comment on
-
Antibody Dynamics, Seroreversion, and Persistence After Severe Acute Respiratory Syndrome Coronavirus 2: Another Answer.Clin Infect Dis. 2021 Dec 16;73(12):2163-2165. doi: 10.1093/cid/ciab243. Clin Infect Dis. 2021. PMID: 33730754 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

