Comparison of pulmonary deposition of nebulized 99m technetium-diethylenetriamine-pentaacetic acid through 3 inhalation devices in healthy dogs
- PMID: 33624851
- PMCID: PMC7995371
- DOI: 10.1111/jvim.16064
Comparison of pulmonary deposition of nebulized 99m technetium-diethylenetriamine-pentaacetic acid through 3 inhalation devices in healthy dogs
Abstract
Background: Inhalation treatment frequently is used in dogs and cats with chronic respiratory disease. Little is known however about the performance of delivery devices and the distribution of aerosolized drugs in the lower airways.
Objective: To assess the performance of 3 delivery devices and the impact of variable durations of inhalation on the pulmonary and extrapulmonary deposition of nebulized 99m technetium-diethylenetriamine-pentaacetic acid (99m Tc-DTPA).
Animals: Ten university-owned healthy Beagle dogs.
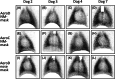
Methods: Prospective crossover study. Dogs inhaled the radiopharmaceutical for 5 minutes either through the Aerodawg spacer with a custom-made nose-muzzle mask, the Aerochamber spacer with the same mask, or the Aerodawg spacer with its original nose mask. In addition, dogs inhaled for 1 and 3 minutes through the second device. Images were obtained by 2-dimensional planar scintigraphy. Radiopharmaceutical uptake was calculated as an absolute value and as a fraction of the registered dose in the whole body.
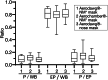
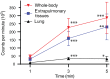
Results: Mean (±SD) lung deposition for the 3 devices was 9.2% (±5.0), 11.4% (±4.9), and 9.3% (±4.6), respectively. Differences were not statistically significant. Uptake in pulmonary and extrapulmonary tissues was significantly lower after 1-minute nebulization, but the mean pulmonary/extrapulmonary deposition ratio (0.38 ± 0.27) was significantly higher than after 5-minute nebulization (0.16 ± 0.1; P = .03). No significant differences were detected after 3- and 5-minute nebulization.
Conclusion and clinical importance: The performance of a pediatric spacer with a custom-made mask is comparable to that of a veterinary device. One-minute nebulization provides lower pulmonary uptake but achieves a better pulmonary/extrapulmonary deposition ratio than does 5-minute nebulization.
Keywords: dog; inhalation; lung; radiopharmaceutical; scintigraphy.
© 2021 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals LLC on behalf of American College of Veterinary Internal Medicine.
Conflict of interest statement
Authors declare no conflict of interest.
Figures




Similar articles
-
Targeted Lung Deposition From Nebulization Is Not Improved in the Lateral Decubitus Position in Healthy Volunteers.Respir Care. 2019 Dec;64(12):1537-1544. doi: 10.4187/respcare.06978. Epub 2019 Sep 10. Respir Care. 2019. PMID: 31506338 Clinical Trial.
-
Investigation of pulmonary deposition of a nebulized radiopharmaceutical agent in awake cats.Am J Vet Res. 2004 Jun;65(6):806-9. doi: 10.2460/ajvr.2004.65.806. Am J Vet Res. 2004. PMID: 15198221
-
Improved efficacy of aerosol delivery to distal airways in pediatric subjects using a new spacer mouth-mask.Eur Ann Allergy Clin Immunol. 2007 Feb;39(2):64-8. Eur Ann Allergy Clin Immunol. 2007. PMID: 17441418 Clinical Trial.
-
Effect of inhaled surfactant on pulmonary deposition and clearance of technetium-99m-DTPA radioaerosol.J Nucl Med. 1998 Mar;39(3):543-7. J Nucl Med. 1998. PMID: 9529308
-
Site of deposition and absorption of an inhaled hydrophilic solute.Br J Clin Pharmacol. 2007 Jun;63(6):722-31. doi: 10.1111/j.1365-2125.2006.02835.x. Epub 2007 Jan 23. Br J Clin Pharmacol. 2007. PMID: 17244190 Free PMC article.
Cited by
-
Use of a Capsaicin Cough Challenge Test to Compare Four Different Techniques for Nebulization Delivery in Cats.Vet Sci. 2024 Jul 17;11(7):320. doi: 10.3390/vetsci11070320. Vet Sci. 2024. PMID: 39058004 Free PMC article.
-
Bacterial Contamination of Inhalation Chambers Used for Cats and Dogs with Chronic Airway Diseases.Pathogens. 2023 Feb 8;12(2):275. doi: 10.3390/pathogens12020275. Pathogens. 2023. PMID: 36839547 Free PMC article.
References
-
- Virchow JC, Crompton GK, Dal Negro R, et al. Importance of inhaler devices in the management of airway disease. Respir Med. 2008;102(1):10‐19. - PubMed
-
- Leemans J, Kirschvink N, Clercx C, Snaps F, Gustin P. Effect of short‐term oral and inhaled corticosteroids on airway inflammation and responsiveness in a feline acute asthma model. Vet J. 2012;192(1):41‐48. - PubMed
-
- Bexfield NH, Foale RD, Davison LJ, Watson PJ, Skelly BJ, Herrtage ME. Management of 13 cases of canine respiratory disease using inhaled corticosteroids. J Small Anim Pract. 2006;47(7):377‐382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

