Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncologic Patients
- PMID: 33625300
- PMCID: PMC7909072
- DOI: 10.1148/radiol.2021210275
Lymphadenopathy in COVID-19 Vaccine Recipients: Diagnostic Dilemma in Oncologic Patients
Abstract
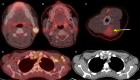
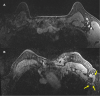
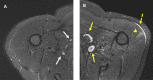
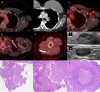
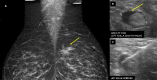
Five cases of axillary lymphadenopathy are presented, which occurred after COVID-19 vaccination and mimicked metastasis in a vulnerable oncologic patient group. Initial radiologic diagnosis raised concerns for metastasis. However, further investigation revealed that patients received COVID-19 vaccinations in the ipsilateral arm prior to imaging. In two cases, lymph node biopsy results confirmed vaccination-related reactive lymphadenopathy. Ipsilateral axillary swelling or lymphadenopathy was reported based on symptoms and physical examination in COVID-19 vaccine trials. Knowledge of the potential for COVID-19 vaccine-related ipsilateral adenopathy is necessary to avoid unnecessary biopsy and change in therapy. © RSNA, 2021.
Figures
References
-
- Moderna COVID-19 Vaccine . US Food & Drug Administration Web site. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise.... Accessed February 06, 2021.
-
- Pfizer-BioNTech COVID-19 Vaccine . US Food & Drug Administration Web site. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise.... Accessed February 06, 2021.
-
- Covid data tracker . US Center for Disease Control and Prevention Web site. https://covid.cdc.gov/covid-data-tracker/#vaccinations. Accessed February 06, 2021.
-
- Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: Moderna COVID-19 Vaccine . US Center for Disease Control and Prevention Web site. https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogeni.... Accessed February 06, 2021.
-
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Jr., Hammitt LL, Tureci O, Nell H, Schaefer A, Unal S, Tresnan DB, Mather S, Dormitzer PR, Sahin U, Jansen KU, Gruber WC, Group CCT. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med 2020;383:2603-2615. doi: 10.1056/NEJMoa2034577 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

