Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19
- PMID: 33625505
- PMCID: PMC7905703
- DOI: 10.1001/jama.2021.2091
Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19
Abstract
Importance: Refinement of criteria for multisystem inflammatory syndrome in children (MIS-C) may inform efforts to improve health outcomes.
Objective: To compare clinical characteristics and outcomes of children and adolescents with MIS-C vs those with severe coronavirus disease 2019 (COVID-19).
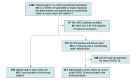
Setting, design, and participants: Case series of 1116 patients aged younger than 21 years hospitalized between March 15 and October 31, 2020, at 66 US hospitals in 31 states. Final date of follow-up was January 5, 2021. Patients with MIS-C had fever, inflammation, multisystem involvement, and positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse transcriptase-polymerase chain reaction (RT-PCR) or antibody test results or recent exposure with no alternate diagnosis. Patients with COVID-19 had positive RT-PCR test results and severe organ system involvement.
Exposure: SARS-CoV-2.
Main outcomes and measures: Presenting symptoms, organ system complications, laboratory biomarkers, interventions, and clinical outcomes. Multivariable regression was used to compute adjusted risk ratios (aRRs) of factors associated with MIS-C vs COVID-19.
Results: Of 1116 patients (median age, 9.7 years; 45% female), 539 (48%) were diagnosed with MIS-C and 577 (52%) with COVID-19. Compared with patients with COVID-19, patients with MIS-C were more likely to be 6 to 12 years old (40.8% vs 19.4%; absolute risk difference [RD], 21.4% [95% CI, 16.1%-26.7%]; aRR, 1.51 [95% CI, 1.33-1.72] vs 0-5 years) and non-Hispanic Black (32.3% vs 21.5%; RD, 10.8% [95% CI, 5.6%-16.0%]; aRR, 1.43 [95% CI, 1.17-1.76] vs White). Compared with patients with COVID-19, patients with MIS-C were more likely to have cardiorespiratory involvement (56.0% vs 8.8%; RD, 47.2% [95% CI, 42.4%-52.0%]; aRR, 2.99 [95% CI, 2.55-3.50] vs respiratory involvement), cardiovascular without respiratory involvement (10.6% vs 2.9%; RD, 7.7% [95% CI, 4.7%-10.6%]; aRR, 2.49 [95% CI, 2.05-3.02] vs respiratory involvement), and mucocutaneous without cardiorespiratory involvement (7.1% vs 2.3%; RD, 4.8% [95% CI, 2.3%-7.3%]; aRR, 2.29 [95% CI, 1.84-2.85] vs respiratory involvement). Patients with MIS-C had higher neutrophil to lymphocyte ratio (median, 6.4 vs 2.7, P < .001), higher C-reactive protein level (median, 152 mg/L vs 33 mg/L; P < .001), and lower platelet count (<150 ×103 cells/μL [212/523 {41%} vs 84/486 {17%}, P < .001]). A total of 398 patients (73.8%) with MIS-C and 253 (43.8%) with COVID-19 were admitted to the intensive care unit, and 10 (1.9%) with MIS-C and 8 (1.4%) with COVID-19 died during hospitalization. Among patients with MIS-C with reduced left ventricular systolic function (172/503, 34.2%) and coronary artery aneurysm (57/424, 13.4%), an estimated 91.0% (95% CI, 86.0%-94.7%) and 79.1% (95% CI, 67.1%-89.1%), respectively, normalized within 30 days.
Conclusions and relevance: This case series of patients with MIS-C and with COVID-19 identified patterns of clinical presentation and organ system involvement. These patterns may help differentiate between MIS-C and COVID-19.
Conflict of interest statement
Figures



Comment in
- doi: 10.1001/jama.2020.10370
References
-
- NYC Health . 2020 Health alert 13: pediatric multi-system inflammatory syndrome potentially associated with COVID-19. Published May 4, 2020. Accessed December 3, 2020. https://www1.nyc.gov/assets/doh/downloads/pdf/han/alert/2020/covid-19-pe...
-
- Centers for Disease Control and Prevention . Emergency preparedness and response: HAN00432. Published May 14, 2020. Accessed December 3, 2020. https://emergency.cdc.gov/han/2020/han00432.asp
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

