Preformulation Characterization of Griffithsin, a Biopharmaceutical Candidate for HIV Prevention
- PMID: 33625602
- PMCID: PMC7903873
- DOI: 10.1208/s12249-021-01931-0
Preformulation Characterization of Griffithsin, a Biopharmaceutical Candidate for HIV Prevention
Abstract
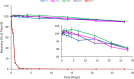
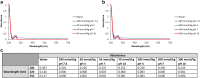
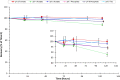
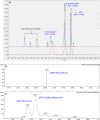
Griffithsin (GRFT) has shown potent anti-HIV activity, and it is being developed as a drug candidate for HIV prevention. Successful implementation requires thorough understanding of its preformulation characterization. In this work, preformulation assessments were conducted to characterize GRFT and identify its degradation pathways under selected conditions of temperature, light, pH, shear, ionic strength, and oxidation. Compatibility with vaginal fluid simulant, vaginal enzymes, Lactobacillus spp., and human cervicovaginal secretions was assessed. The purity, melting temperature, and HIV gp120-binding affinity of GRFT stored at 4°C and 25°C in phosphate-buffered saline (PBS) were assessed for 2 years. Chemical modifications were evaluated by intact mass analysis and peptide sequencing. Excised human ectocervical tissue permeability and localization of GRFT were evaluated. Our results demonstrated GRFT to be safe and stable under all the preformulation assessment conditions studied except oxidative stress. When GRFT was exposed to hydrogen peroxide or human cervicovaginal secretion, methionine 78 in the protein sequence underwent oxidation. GRFT did not permeate through human cervical tissue but adhered to the superficial epithelial tissue. The 2-year stability study revealed no significant change in GRFT's aggregation, degradation, melting temperature, or gp120-binding affinity despite a slow increase in oxidation over time. These studies elucidated desirable safety and bioactivity profile for GRFT, showing promise as a potential drug candidate for HIV prevention. However, susceptibility to oxidative degradation was identified. Effective protection of GRFT from oxidation is required for further development.
Keywords: GRFT; HIV prevention; griffithsin; oxidation; preformulation.
Figures



References
-
- UNAIDS. Fact Sheet - Global AIDS Update 2019 2019 [Available from: https://www.unaids.org/en/resources/fact-sheet.
-
- O'Keefe BR, Vojdani F, Buffa V, Shattock RJ, Montefiori DC, Bakke J, Mirsalis J, d'Andrea AL, Hume SD, Bratcher B, Saucedo CJ, McMahon JB, Pogue GP, Palmer KE. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc Natl Acad Sci U S A. 2009;106(15):6099–6104. doi: 10.1073/pnas.0901506106. - DOI - PMC - PubMed
-
- Barton C, Kouokam JC, Lasnik AB, Foreman O, Cambon A, Brock G, Montefiori DC, Vojdani F, McCormick AA, O'Keefe BR, Palmer KE. Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models. Antimicrob Agents Chemother. 2014;58(1):120–127. doi: 10.1128/AAC.01407-13. - DOI - PMC - PubMed
-
- Alexandre KB, Gray ES, Lambson BE, Moore PL, Choge IA, Mlisana K, Karim SSA, McMahon J, O'Keefe B, Chikwamba R, Morris L. Mannose-rich glycosylation patterns on HIV-1 subtype C gp120 and sensitivity to the lectins, Griffithsin. Cyanovirin-N and Scytovirin. Virology. 2010;402(1):187–196. doi: 10.1016/j.virol.2010.03.021. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

