Growth and Differentiation of Circulating Stem Cells After Extensive Ex Vivo Expansion
- PMID: 33625723
- PMCID: PMC8169750
- DOI: 10.1007/s13770-021-00330-7
Growth and Differentiation of Circulating Stem Cells After Extensive Ex Vivo Expansion
Abstract
Background: Stem cell therapy is gaining momentum as an effective treatment strategy for degenerative diseases. Adult stem cells isolated from various sources (i.e., cord blood, bone marrow, adipose tissue) are being considered as a realistic option due to their well-documented therapeutic potentials. Our previous studies standardized a method to isolate circulating multipotent cells (CMCs) that are able to sustain long term in vitro culture and differentiate towards mesodermal lineages.
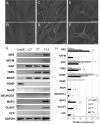
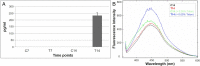
Methods: In this work, long-term cultures of CMCs were stimulated to study in vitro neuronal and myogenic differentiation. After induction, cells were analysed at different time points. Morphological studies were performed by scanning electron microscopy and specific neuronal and myogenic marker expression were evaluated using RT-PCR, flow cytometry and western blot. For myogenic plasticity study, CMCs were transplanted into in vivo model of chemically-induced muscle damage.
Results: After neurogenic induction, CMCs showed characteristic dendrite-like morphology and expressed specific neuronal markers both at mRNA and protein level. The calcium flux activity of CMCs under stimulation with potassium chloride and the secretion of noradrenalin confirmed their ability to acquire a functional phenotype. In parallel, the myogenic potential of CMCs was confirmed by their ability to form syncytium-like structures in vitro and express myogenic markers both at early and late phases of differentiation. Interestingly, in a rat model of bupivacaine-induced muscle damage, CMCs integrated within the host tissue taking part in tissue repair.
Conclusion: Overall, collected data demonstrated long-term cultured CMCs retain proliferative and differentiative potentials suggesting to be a good candidate for cell therapy.
Keywords: Circulating stem cells; Degenerative diseases; Myogenesis; Neurogenesis; Regenerative medicine.
Conflict of interest statement
The Authors declare that there is no conflict of interest regarding the publication of this article.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
