Retinotopic variations of the negative blood-oxygen-level dependent hemodynamic response function in human primary visual cortex
- PMID: 33625949
- PMCID: PMC8282228
- DOI: 10.1152/jn.00676.2020
Retinotopic variations of the negative blood-oxygen-level dependent hemodynamic response function in human primary visual cortex
Abstract
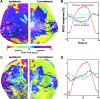
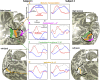
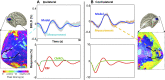
Functional magnetic resonance imaging (fMRI) measures blood-oxygen-level-dependent (BOLD) contrast that is generally assumed to be linearly related to excitatory neural activity. The positive hemodynamic response function (pHRF) is the positive BOLD response (PBR) evoked by a brief neural stimulation; the pHRF is often used as the impulse response for linear analysis of neural excitation. Many fMRI studies have observed a negative BOLD response (NBR) that is often associated with neural suppression. However, the temporal dynamics of the NBR evoked by a brief stimulus, the negative HRF (nHRF), remains unclear. Here, a unilateral visual stimulus was presented in a slow event-related design to elicit both pHRFs in the stimulus representation (SR), and nHRFs elsewhere. The observed nHRFs were not inverted versions of the pHRF previously reported. They were characterized by a stronger initial negative response followed by a significantly later positive peak. In contralateral primary visual cortex (V1), these differences varied with eccentricity from the SR. Similar nHRFs were observed in ipsilateral V1 with less eccentricity variation. Experiments with the blocked version of the same stimulus confirmed that brain regions presenting the unexpected nHRF dynamics correspond to those presenting a strong NBR. These data demonstrated that shift-invariant temporal linearity did not hold for the NBR while confirming that the PBR maintained rough linearity. Modeling indicated that the observed nHRFs can be created by suppression of both blood flow and oxygen metabolism. Critically, the nHRF can be misinterpreted as a pHRF due to their similarity, which could confound linear analysis for event-related fMRI experiments.NEW & NOTEWORTHY We investigate dynamics of the negative hemodynamic response function (nHRF), the negative blood-oxygen-level-dependent (BOLD) response (NBR) evoked by a brief stimulus, in human early visual cortex. Here, we show that the nHRFs are not inverted versions of the corresponding pHRFs. The nHRF has complex dynamics that varied significantly with eccentricity. The results also show shift-invariant temporal linearity does not hold for the NBR.
Keywords: hemodynamic response function; linearity; neural suppression; neurovascular coupling; primary visual cortex.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

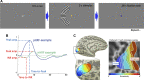
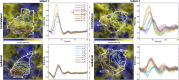
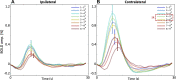
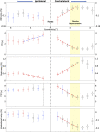
References
Publication types
MeSH terms
Grants and funding
- R01 NS095933/NS/NINDS NIH HHS/United States
- R56 NS095933/NS/NINDS NIH HHS/United States
- K25 HL131997/HL/NHLBI NIH HHS/United States
- R01NS95933 S1/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01NS95933/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

