Ultra-rapid detection of SARS-CoV-2 in public workspace environments
- PMID: 33626039
- PMCID: PMC7904170
- DOI: 10.1371/journal.pone.0240524
Ultra-rapid detection of SARS-CoV-2 in public workspace environments
Abstract
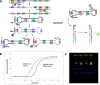
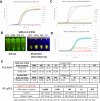
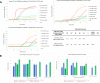
Managing the pandemic caused by SARS-CoV-2 requires new capabilities in testing, including the possibility of identifying, in minutes, infected individuals as they enter spaces where they must congregate in a functioning society, including workspaces, schools, points of entry, and commercial business establishments. Here, the only useful tests (a) require no sample transport, (b) require minimal sample manipulation, (c) can be performed by unlicensed individuals, (d) return results on the spot in much less than one hour, and (e) cost no more than a few dollars. The sensitivity need not be as high as normally required by the FDA for screening asymptomatic carriers (as few as 10 virions per sample), as these viral loads are almost certainly not high enough for an individual to present a risk for forward infection. This allows tests specifically useful for this pandemic to trade-off unneeded sensitivity for necessary speed, simplicity, and frugality. In some studies, it was shown that viral load that creates forward-infection risk may exceed 105 virions per milliliter, easily within the sensitivity of an RNA amplification architecture, but unattainable by antibody-based architectures that simply target viral antigens. Here, we describe such a test based on a displaceable probe loop amplification architecture.
Conflict of interest statement
Firebird Biomolecular Sciences, LLC, GenePath Diagnostics, Inc., and GenePath Diagnostics India Pvt. Ltd. employ the indicated authors and the specific roles of these authors are articulated in the ‘author contributions’ section. OY, ZY, SAB and their institutions own intellectual property associated with the assay. Some of the items mentioned here are sold by Firebird Biomolecular Sciences, LLC, which employs the indicated authors and is owned by SAB. This does not alter the authors’ and institutions’ adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- Riou J, Althaus CL. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2020;25(4):2000058 10.2807/1560-7917.ES.2020.25.4.2000058 . - DOI - PMC - PubMed
-
- Benner SA, Gaucher EA, Li T. Post-genomic evolutionary analyses of the severe acute respiratory syndrome (SARS) virus genome using the Master Catalog[R] interpretive proteomics platform. PharmaGenomics. 2003;3:S17.
-
- Chan JF, Lau SK, To KK, Cheng VC, Woo PC, Yuen KY. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev 2015;28(1098–6618 (Electronic)):465–522. doi: D—NLM: PMC4402954 [Available on 04/01/16] EDAT- 2015/03/27 06:00 MHDA- 2015/04/22 06:00 CRDT- 2015/03/27 06:00 PMCR- 2016/04/01 00:00 AID—28/2/465 [pii] AID—10.1128/CMR.00102-14 PST—ppublish. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

