Real-world management of hyperphosphataemia with sucroferric oxyhydroxide: the VELREAL multicentre study
- PMID: 33626111
- PMCID: PMC7886585
- DOI: 10.1093/ckj/sfaa226
Real-world management of hyperphosphataemia with sucroferric oxyhydroxide: the VELREAL multicentre study
Abstract
Background: The efficacy and safety of sucroferric oxyhydroxide (SO) have been reported in clinical trials. However, real-life data are scarce. This study presents data on the use, efficacy and safety of SO in real clinical practice.
Methods: We performed a retrospective multicentre study, without any influence on the prescription decisions, that included 220 patients from 11 Spanish centres. Demographic, treatment, analytical and nutritional parameters and adherence, side effects and dropout rates were collected during 6 months.
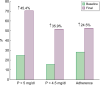
Results: SO was initiated due to inadequate control of serum phosphate (P) in 70% of participants and in 24.5% to reduce the number of tablets. Monotherapy with SO increased from 44% to 74.1%, with a reduction in the average daily number of sachets/tablets from six to two. Serum P decreased by 20% (4.6 ± 1.2 versus 5.8 ± 1.3 mg/dL; P < 0.001), with a significant reduction in intact parathyroid hormone levels (P < 0.01). The percentage of patients with adequate serum P control at threshold levels of 5 and 4.5 mg/dL increased by 45.4% and 35.9%, respectively. Serum ferritin was not modified, while the transferrin saturation index increased significantly (P = 0.04). Serum albumin and normalized protein catabolic rate, when normalized by serum P, increased, averaging 37% and 39%, respectively (P < 0.001). Adherent patients increased from 28.2% to 52.7%. Adverse effects were reported by 14.1% of participants, with abandonment of treatment in 9.5%.
Conclusions: The use of SO in real-life results in better control of serum P, a reduction in the number of tablets and an improvement in therapeutic adherence. In addition, it may be beneficial with regards to secondary hyperparathyroidism and nutritional status.
Keywords: haemodialysis; hyperphosphataemia; nutritional status; sucorferric oxyhydroxide; therapeutic adherence.
© The Author(s) 2021. Published by Oxford University Press on behalf of ERA-EDTA.
Figures
Similar articles
-
Efficacy and safety of sucroferric oxyhydroxide compared with sevelamer hydrochloride in Japanese haemodialysis patients with hyperphosphataemia: A randomized, open-label, multicentre, 12-week phase III study.Nephrology (Carlton). 2017 Apr;22(4):293-300. doi: 10.1111/nep.12891. Nephrology (Carlton). 2017. PMID: 27496336 Free PMC article. Clinical Trial.
-
Efficacy and safety of sucroferric oxyhydroxide in the treatment of hyperphosphataemia in chronic kidney disease. FOSFASTUR study.Nefrologia (Engl Ed). 2021 Jan-Feb;41(1):45-52. doi: 10.1016/j.nefro.2020.06.008. Epub 2020 Nov 22. Nefrologia (Engl Ed). 2021. PMID: 33239181 English, Spanish.
-
Efficacy and safety of sucroferric oxyhydroxide in the treatment of hyperphosphataemia in chronic kidney disease in Asturias. FOSFASTUR study.Nefrologia (Engl Ed). 2021 Jan-Feb;41(1):45-52. doi: 10.1016/j.nefroe.2021.02.005. Epub 2021 Mar 1. Nefrologia (Engl Ed). 2021. PMID: 36165361
-
Sucroferric oxyhydroxide for hyperphosphatemia: a review of real-world evidence.J Nephrol. 2022 Apr;35(3):875-888. doi: 10.1007/s40620-021-01241-5. Epub 2022 Feb 9. J Nephrol. 2022. PMID: 35138627 Free PMC article. Review.
-
Sucroferric oxyhydroxide: a review in hyperphosphataemia in chronic kidney disease patients undergoing dialysis.Drugs. 2015 Apr;75(5):533-42. doi: 10.1007/s40265-015-0366-1. Drugs. 2015. PMID: 25761962 Review.
Cited by
-
Do we need new phosphate binders in dialysis?Clin Kidney J. 2020 Dec 16;14(2):474-475. doi: 10.1093/ckj/sfaa246. eCollection 2021 Feb. Clin Kidney J. 2020. PMID: 33626110 Free PMC article.
References
-
- Djukanović L, Dimković N, Marinković J. et al. Association between hemodialysis patient outcomes and compliance with KDOQI and KDIGO targets for mineral and bone metabolism. Nephron 2016; 132: 168–174 - PubMed
-
- Tonelli M, Pannu N, Manns B.. Oral phosphate binders in patients with kidney failure. N Engl J Med 2010; 362: 1312–1324 - PubMed
-
- Sprague SM, Floege J.. Sucroferric oxyhydroxide for the treatment of hyperphosphatemia. Expert Opin Pharmacother 2018; 19: 1137–1148 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

