Clinical impact of post-progression survival on overall survival in patients receiving nivolumab monotherapy as a second-line treatment for advanced non-small cell lung cancer
- PMID: 33626218
- PMCID: PMC8046034
- DOI: 10.1111/1759-7714.13886
Clinical impact of post-progression survival on overall survival in patients receiving nivolumab monotherapy as a second-line treatment for advanced non-small cell lung cancer
Abstract
Background: The effect of second-line treatment on overall survival (OS) may be affected by subsequent treatment in patients with non-small cell lung cancer (NSCLC); however, in such patients, the correlation between post-progression survival (PPS) and OS is unclear. Our study assessed the correlation of progression-free survival (PFS) and PPS with OS, using individual patient data, in advanced NSCLC patients who were treated with second-line nivolumab monotherapy, METHODS: Between January 2016 and March 2019, we evaluated 92 NSCLC patients who received second-line nivolumab treatment after first-line platinum-based combination chemotherapy. Using individual patient data, the correlations of PFS and PPS with OS were examined.
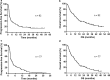
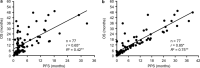
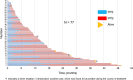
Results: Linear regression and Spearman rank correlation analysis demonstrated that PPS was strongly correlated with OS (r = 0.85, p < 0.05, R2 = 0.75), while PFS was moderately correlated with OS (r = 0.65, p < 0.05, R2 = 0.42). Performance status at the beginning of second-line treatment, immune checkpoint inhibitor rechallenge, and the number of treatment regimens used post-progression, after the second-line treatment significantly correlated with PPS (p < 0.05). In advanced NSCLC patients who underwent second-line treatment with nivolumab, in comparison to PFS, there was a stronger correlation between PPS and OS.
Conclusions: Our findings suggest that subsequent treatment for disease progression after a second-line nivolumab treatment had a significant impact on OS.
Keywords: nivolumab; non-small cell lung cancer; survival.
© 2021 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
None of the authors have any financial or personal relationships with other people or organizations that could inappropriately influence this work.
Figures

Similar articles
-
Post-Progression Survival Associated with Overall Survival in Patients with Advanced Non-Small-Cell Lung Cancer Receiving Docetaxel Monotherapy as Second-Line Chemotherapy.Chemotherapy. 2017;62(4):205-213. doi: 10.1159/000456534. Epub 2017 Apr 6. Chemotherapy. 2017. PMID: 28380484
-
Individual-level data on the relationships of progression-free survival and post-progression survival with overall survival in patients with advanced non-squamous non-small cell lung cancer patients who received second-line chemotherapy.Med Oncol. 2014 Aug;31(8):88. doi: 10.1007/s12032-014-0088-3. Epub 2014 Jun 25. Med Oncol. 2014. PMID: 24961467 Clinical Trial.
-
Evaluation of the efficacy and safety of nivolumab in the second- or later-line treatment of patients with locally advanced/metastatic non-small cell lung cancer in Türkiye: a retrospective multicenter non-interventional registry study.Curr Med Res Opin. 2024 Jul;40(7):1171-1178. doi: 10.1080/03007995.2024.2359026. Epub 2024 Jun 22. Curr Med Res Opin. 2024. PMID: 38809230
-
Postprogression survival for first-line chemotherapy of patients with advanced non-small-cell lung cancer.Ann Oncol. 2012 Jun;23(6):1537-41. doi: 10.1093/annonc/mdr487. Epub 2011 Oct 29. Ann Oncol. 2012. PMID: 22039091 Review.
-
Clinical significance of post-progression survival in lung cancer.Thorac Cancer. 2017 Sep;8(5):379-386. doi: 10.1111/1759-7714.12463. Epub 2017 Jun 19. Thorac Cancer. 2017. PMID: 28627767 Free PMC article. Review.
References
-
- Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363–85. - PubMed
-
- Soria JC, Massard C, Le Chevalier T. Should progression‐free survival be the primary measure of efficacy for advanced NSCLC therapy? Ann Oncol. 2010;21:2324–32. - PubMed
-
- Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first‐line therapy for nonsquamous non‐small‐cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–34. - PubMed
-
- Saad ED, Katz A, Buyse M. Overall survival and post‐progression survival in advanced breast cancer: a review of recent randomized clinical trials. J Clin Oncol. 2010;28:1958–62. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

